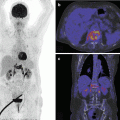
Fig. 26.1
I-MRA of the left hip. Synovial osteochondromatosis with loose bodies (arrow) and hydrops
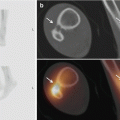
Fig. 26.2
I-MRA of the right hip. Mixed cam and pincer configuration in a 34-year-old female. There is an obvious bump (cam) on the anterior femoral neck (big arrow) with alpha angle over 50° (striped curved line). The acetabulum is overcovering the femoral head (small arrows); the straight line connecting the anterior and posterior margin of the labrum at the midsection of the femur head is located latero-anterior to the center of the femur head (pincer)
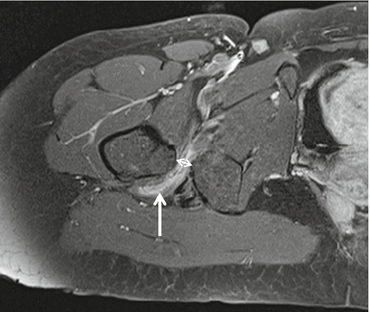
Fig. 26.3
I-MRA of the right hip. Reduced distance between the ischial tuberosity on the left and lesser trochanter on the right to 1 cm (normally 2 cm) (double arrow) with edema in the quadratus femoris muscle (arrow). I-MRA T1 FS axial image, with high SI in the muscle: edema with contrast enhancement
Superficial soft tissues are demonstrated on US with the use of high-frequency probes at a much higher spatial resolution compared to MRI. The deeper the structures are located, the lower the ultrasound frequency and the lower the spatial resolution. Ultrasound is used in the initial assessment of evaluation of joint fluid and for diagnostic evaluation of tendons and muscles abnormalities including tendon snapping (Dawes and Seidenberg 2014). The use of US can be the most appropriate way to examine the postoperative hip as it has little artifacts from metal in hip prosthesis. It can be used in the evaluation of the superficial-located peripheral nerves (NCFL, femoral nerve, and sciatic nerve) with high resolution using high-frequency probes.
Ultrasound can also be used to guide injections: injection for MR or CT arthrography, diagnostic or therapeutic aspiration, diagnostic injection with anesthetics, or therapeutic injection (Jacobson et al. 2012). US is preferred for the examination of the pediatric hip and pelvis, because of the lack of radiation.
26.3 Pathology
26.3.1 Intra-articular Hip Pathology
Bony morphology of the hip joint is primary examined radiographically. Intra-articular pathology of the hip is ideally assessed after administration of intra-articular diluted gadolinium (1/200 solution). D-MRA has an accuracy as high as 90 % in the diagnosis of labral tear (Perdikakis et al. 2011). D-MRA allows to recognize anatomic variants like intra-articular synovial plicae and sublabral sulci. Causes of labral lesions are femoroacetabular impingement (FAI), trauma, hip dysplasia, capsular laxity, degenerative joint disease, and recently described iliopsoas impingement. Paralabral cysts are a hallmark of a labral tear; they are easily identified because of their liquid content, often in multilobular cysts that may fill with intra-articular contrast material (Magee and Hinson 2000). The cysts are best depicted on the fluid-sensitive sequences and can be missed on the T1 FS sequences as they often do not fill with gadolinium contrast.
Hyaline articular cartilage abnormalities also may be identified but with lower sensitivity compared with labral tears (Perdikakis et al. 2011). Recent prospective studies demonstrate comparable accuracy of I-MRA compared to D-MRA at 3 T (Petchprapa et al. 2015).
Features of osteoarthritis are joint space narrowing, osteophyte formation, variable reactive BME, and subchondral cysts.
Hydrops can be present and is well evaluated on US or MRI (MRI or I-MRA, not on D-MRA since the joint is injected). Intra-articular loose bodies are well demonstrated on MRA (direct or indirect) (Fig. 26.1). Intra-articular loose bodies may give a snapping sensation in the hip with movement (snapping hip).
AVN of the hip affects mostly middle-aged patients in their active phase of living. It may occur in athletes especially after subcapital fracture or hip dislocation leading to vascular disruption of the femoral head. D-MRA is an accurate method to detect tears of the ligamentum teres as a cause of vascular disruption of the femoral head (Chang et al. 2015). Early diagnosis of AVN plays a crucial role with respect to therapeutic success and prognosis. MRI is very sensitive and specific in the diagnosis of AVN of the hip. Although bone scintigraphy and CT and more recently PET have been used for diagnosing AVN, currently the most important imaging methods included in the most used classification systems for AVN of the hip (Steinberg, Ficat and ARCO) are radiographs and MRI (Karantanas 2013). Radiographs are normal in the early non-symptomatic stages of AVN. Specific crescent sign on radiographs, representing subchondral parallel bone fracture, is an early sign of collapse and found in symptomatic more advanced AVN stages (Fig. 26.4). MRI is superior to plain radiographs in depicting the early stages, in staging accurately the lesions that result in early articular collapse, and in monitoring vascularized fibular grafts. A specific and very early (pre-collapse) MRI sign of AVN, found in 100 % of cases, is a low SI demarcation zone on T1- and T2WI extending from subchondral bone to subchondral bone circumscribing the necrotic portion (Fig. 26.5a, b). Pathognomonic for AVN is the double line at the interface between necrotic and healthy bone on T2WI with high signal on the necrotic side representing reparative tissue and low SI line at the vital part representing sclerosis and fibrosis (Fig. 26.5b). The double-line sign is present in 65–80 % of AVN (Mitchell et al. 1987). The reported sensitivity of MRI for early diagnosis of osteonecrosis ranges between 88 and 100 % compared with 81 % of bone scintigraphy (Karantanas 2013). The necrosis is most often located in the anterosuperior weightbearing region. The treatment of FHAVN is determined in large part by the stage of the disease and by the location and size of the necrotic fragment. The capital point of all the classification systems is the loss of the spherical surface of the femoral head representing a point of no return. The diagnosis of a non-spherical articular surface is based only on plain radiographs in all classification systems currently used. MRI is used only at the early pre-collapse stages. Especially in the pre-collapse stage, calculation of size in percentage of FH involved and the location of the lesion are the most significant factors predicting the possibility of collapse and thus the prognosis.
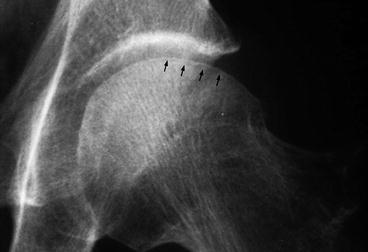
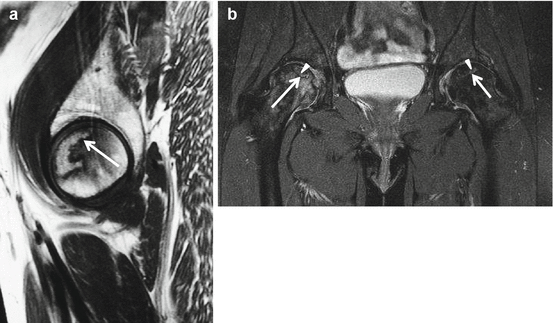

Fig. 26.4
Radiograph of the left hip in a patient with AVN. Crescent line (arrows) is demonstrated at the latero-superior subchondral area. No obvious collapse of the femur head is demonstrated

Fig. 26.5
MRI of AVN of the hip. (a) Sagittal T1WI of the left hip and (b) coronal T2WI with FS of both hips with bilateral AVN in another patient. Incidental finding in a, pain in the right hip region was reported in patient b. Demarcation (arrows) zone circumscribing the necrotic portion on T1- and T2WI. Pathognomonic double-line sign on T2WI (b arrowheads pointing at the high SI line representing reparative tissue). Normal subchondral bone lamella is present in a. On T2WI perilesional BME is detected in patients with loss of spherical surface of the femoral head related to collapse of the subchondral lamella (b right hip); no perilesional BME is present on the left side
Based on the shape and subchondral location of the lesion, AVN can be differentiated from subchondral insufficiency fractures (SIF) in osteoporotic women. The AVN lesion has a smooth delineation, is concave to the articular surface, and circumscribes the entire necrotic segment. In insufficiency fractures the delineation of the fracture is irregular, discontinuous, and convex or parallel to the articular surface. Subchondral fatigue fractures in young athletes are rare but show identical imaging findings as the insufficiency fractures and may be located either in the femoral head or in the acetabulum (Fig. 26.6) (Yoon et al. 2012). Joint effusion and bone marrow edema (BME) are found and precede the fracture line in fatigue fractures.
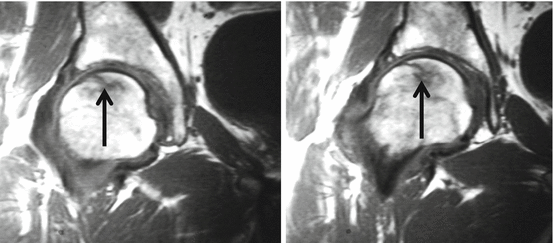

Fig. 26.6
MRI of subchondral fatigue fracture of the right hip. Coronal T1WI with irregular fracture line arrows at the subchondral area without complete circumscription to the subchondral lamella. Area of reduced signal surrounds the fracture line and represents BME
As stated before only radiographs are employed routinely for the evaluation of collapse. It has been shown that plain radiographs overestimate stage II (pre-collapse) and underestimate stage III (collapse before flattening) lesions and are inaccurate in estimating the collapse size, which is an important parameter in therapeutic decisions. Therefore, it has been suggested that the wider use of MRI findings in staging AVN may be necessary (Karantanas 2013).
26.3.2 Femoroacetabular Impingement
Femoroacetabular impingement (FAI) was first described in 1999. FAI is the abutment of the femur with the acetabular rim causing degeneration of the labrum and cartilage. Two types of FAI are described. Cam impingement is caused by a non-spherical head resulting in an osseous bump lateral or anterosuperior on the femoral neck. With flexion and internal rotation at the hip, abutment may proceed with damage to the anterosuperior cartilage and tearing off of the labrum. It is more frequent in males. The second type is pincer impingement, caused by acetabular overcoverage. Osseous impaction along the anterosuperior or superior femoral neck with compression may proceed with damage of the anterosuperior labrum and a small rim of chondromalacia. Posteroinferior cartilage lesions are caused by “contrecoup” forces in pincer-type impingement. Pincer type is more frequent in females. Most of the FAI are mixed cam and pincer impingement (Fig. 26.2). FAI has been shown to be responsible for a large proportion of tears of the acetabular labrum, a major cause of hip pain in young adults. It is now established that FAI is a major factor in the development of early osteoarthritis of the hip in athletes (soccer players) but also in the general population.
A number of conditions are associated with FAI. Developmental hip dysplasia (DHD), Legg–Calve–Perthes disease (LCP), slipped capital femoral epiphysis (SCFE), and malunited fractures of the femoral neck are all anatomical abnormalities related to FAI. Variant anatomy of the femoral head and acetabulum responsible for FAI is depicted on radiographs (Figs. 26.7a–c and 26.9b) (Scheidt et al. 2014) or MRI–MRA in paracoronal series parallel to the femur neck (Fig. 26.8a, c). In patients with cam and/or pincer morphology, repetitive adduction with flexion of the hip causes impingement of labrum and cartilage with lesions as a result. Specific clinical impingement test that may elucidate pain is done with flexion, adduction, and internal rotation of the hip (FADDIR). These labrum and cartilage lesions are evaluated with MRA or CTA (Fig. 26.8a–d); high-magnetic-field MRI at 3 Tesla (T) is capable of demonstrating lesions without gadolinium administration. In pincer-type impingement, the labrum is crushed and torn at the free lateral margin; calcifications and ossifications at the labrum may develop; a narrow strip of chondromalacia is found at the level of the labral lesion; a broader area of chondromalacia may be found at the posterior part of the hip joint related to lever action of the femur metaphysis at the anterior acetabulum. Cam femur morphology typically leads to push-off lesions of the labrum relative to the limbus acetabula with dissociation of the labro-cartilaginous area and development of cystic expansion through the dissociation (paralabral cyst). Labral lesions are not accurately depicted on US; paralabral cyst may be the only US sign of labral dissociation.
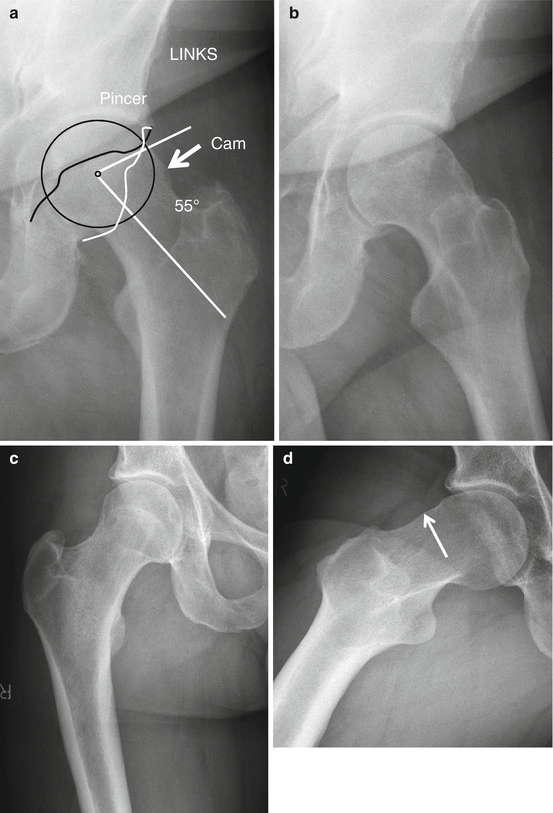
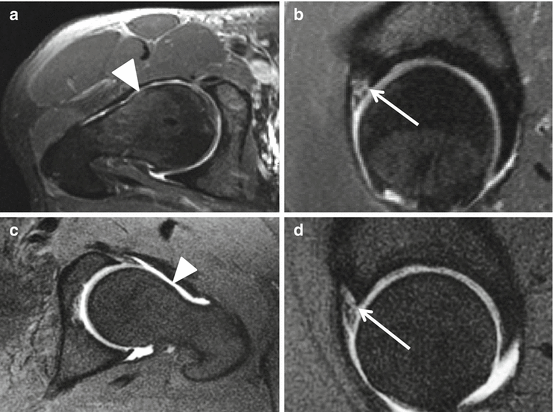
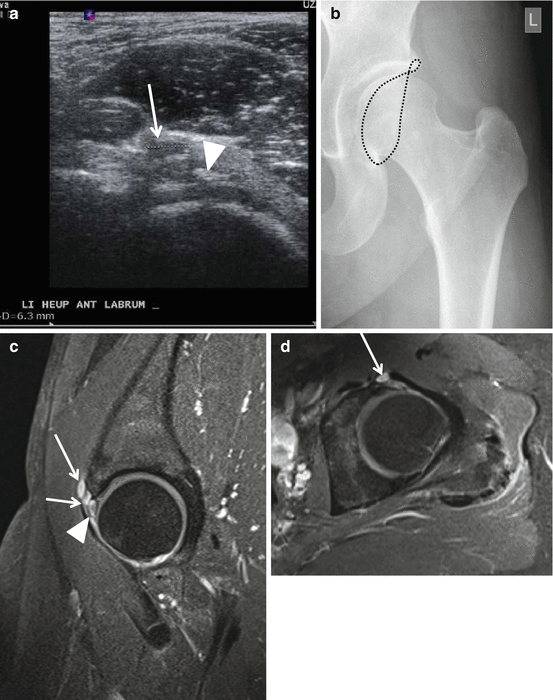

Fig. 26.7
Radiographic evaluation of FAI-related osseous anomalies. (a, b) AP view (a) and Lequesne false profile (b) of the left hip in a patient with combined cam (arrow) and pincer morphology demonstrated on AP view (a). (c, d) AP view (c) and Dunn view (d) in a 44-year-old patient with positive impingement test at the right hip with demonstration of small cam (arrow) on Dunn view (d) that is not demonstrated on AP view (c). Generally three views, AP pelvis (a, image cutout on the right hip), Lequesne false profile (b), and Dunn view (d different patient), are convenient to define femur and acetabular anatomy. The most convenient technique to obtain a digital Dunn view (d) is prone patient position, with hip in 45° flexion and with 20° abduction and non-inclined AP radiation bundle centered on the hip joint. Cam morphology is objectified by calculation of an alpha angle of over 50° on AP view of the pelvis (a white angle) and/or Dunn view (d). Related to the anterolateral location of cam, the AP view is less sensitive in the detection of cam morphology compared to Dunn view. Additional Dunn view is not needed if cam is depicted on AP view (patient a, b). Pincer morphology is depicted on AP pelvis when a crossover is depicted of the ischial spine (anterior limbus of the acetabulum) (a black irregular line) and the posterior acetabular limbus (a white irregular line). On Lequesne false profile, joint space narrowing at the posterior part of the joint is depicted

Fig. 26.8
MRA of labral dissociation, comparison of I-MRA and D-MRA in different patients. I-MRA with axial (a) and sagittal (b) series. D-MRA with axial (c) and sagittal (d) series. Cam-type femur is documented on a and c (arrowheads). Gadolinium interposition between limbus acetabuli and labrum at the anterolateral part is depicted on sagittal images (b, d arrows). Increased expansion in D-MRA (c, d) compared to I-MRA is obvious (a, b)

Fig. 26.9
Radiograph, US, and MRI of labral degeneration and paralabral cyst. Twenty-eight-year-old female with chronic pain at the left hip. US sagittal view (a) demonstrating paralabral cyst (arrow) and calcifications at the labrum (arrowhead). Radiographic AP view with demonstration of limbus acetabuli lining with crossover related to pincer type (b). I-MRA (c sagittal section and d axial section) confirmed the paralabral cyst (arrows) and also demonstrated cystic degeneration of the labrum (arrowhead)
26.3.3 Other Impingement Syndromes Around the Hip
Ischiofemoral impingement syndrome (Fig. 26.3) can be the cause of hip pain due to impingement of the quadratus femoris muscle between the ischium and femur. It is usually associated with previous trauma, mass lesions, or surgery. MRI and CT detect a reduced distance between the ischial tuberosity and lesser trochanter (normal ≥ 2 cm), and on fluid-sensitive sequences on MRI, increased signal intensity of the quadratus femoris muscle is detected; positioning with MRI in full range of motion improves detection (Torriani 2009; Singer et al. 2014).
Iliopsoas impingement is a recently described entity in the orthopedic literature. It can be the cause of labral tears, especially those located in the 3-o’clock position. This type of impingement is an arthroscopic diagnosis and treated with arthroscopic iliopsoas tenotomy at labral level. The only reliable MR feature seems to be the visualization of a labral tear at the 3-o’clock position on MR arthrography (Blankenbaker 2012).
26.3.4 Snapping Hip
The prevalence of snapping hip or coxa saltans is estimated to occur in up to 10 % of the general population, but it is especially seen in athletes such as dancers, soccer players, weight lifters, and runners. The cause of snapping hip can be intra- or extra-articular.
Two types of extra-articular snapping hip are known: anterior located snapping iliopsoas tendon and lateral located snapping iliotibial band. Lateral snapping hip is attributed to the abrupt movement of the iliotibial band across the greater trochanter. Anterior snapping hip or snapping of the iliopsoas tendon usually requires contraction of the hip flexors and may be difficult to differentiate from intra-articular causes of snapping (Lewis 2010). A specific but not sensitive examination to evaluate snapping iliopsoas tendon is dynamic ultrasound performed by an experienced ultrasonographist. With regard to snapping iliopsoas, the transducer is placed parallel to the inguinal ligament over the iliopsoas at the level of the bony pelvis. Dynamic US examination is performed, the patient is asked to flex and externally rotate the hip in a frog-leg position, and then US examination is performed during slow straightening of the leg. In the abnormal situation, medial fibers of the iliacus muscle are interposed between the psoas major tendon and the ilium. Lateral snapping hip is caused by snapping of the iliotibial tract or gluteus maximus relative to the greater trochanter. Intra-articular processes may produce a snapping hip sensation, such as intra-articular bodies (Magee and Hinson 2000; Lee et al. 2013).
26.3.5 Bursae/Bursitis
A distended iliopsoas bursa is located anterior to the femoral head and medial to the iliopsoas tendon; a communication with the joint can be depicted in 15 % of the normal population and is increasingly found in cases of hip disease or hip prosthesis. It can extend deep or superficial to the iliopsoas muscle and extend far intra-abdominally (Blankenbaker 2008). US is sensitive to demonstrate the superficially located iliopsoas bursa (Figs. 26.10 and 26.11a–c).
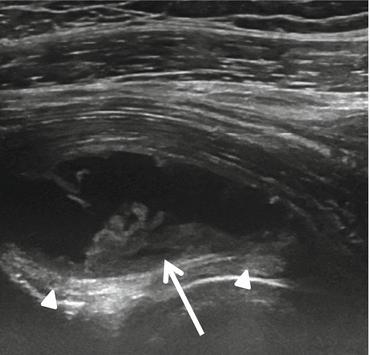
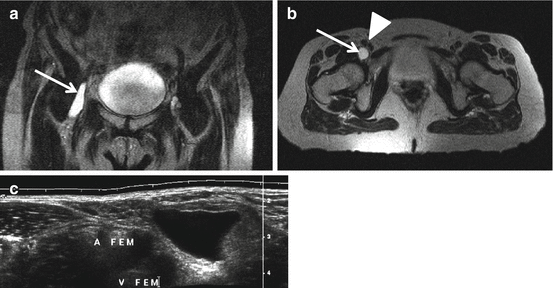

Fig. 26.10
Iliopsoas bursitis. US sagittal plane at the level of the iliofemoral ligament (arrowhead) shows an iliopsoas bursitis with synovial proliferation (arrow)

Fig. 26.11
Iliopsoas bursitis MRI and US in two different patients. Coronal (a) and axial (b) T2WI with high-intensity elongated bursa (arrow) along the course of the psoas MTJ lateral to the femoral artery and vein (arrowhead). Axial US (c) demonstration of assonant bursa in between the iliopsoas and femoral artery and vein
MRI is the modality of choice for deeper located bursitis like obturator externus bursitis and ischiogluteal bursitis. Obturator externus bursitis may cause groin pain. MRI demonstrates the bursa between the obturator externus muscle and the ischium.
Ischiogluteal bursitis is also best documented on MRI since it is located deep to the gluteus muscles and posteroinferior to the ischial tuberosity. The superior end of the bursal sac abuts the inferomedial aspect of the ischial tuberosity (Kil-Ho Cho et al. 2004).
Trochanteric bursitis can be found in case of trochanteric pain syndrome, especially in case of tendinopathy of the gluteus tendons as described below in Sect. 26.3.6.
26.3.6 Greater Trochanter Pain Syndrome
Greater trochanteric pain syndrome (GTPS) is mostly caused by the gluteus medius and minimus pathology: tendinosis, tendinous calcification, partial tendon tears, or complete tendon rupture. Fluid in the bursae is often not prominent and secondary to tendinopathy. Histologically no inflammation is found in the distended trochanteric bursae (Silva et al. 2008), and the distended bursae itself are not associated with pain (Blankenbaker 2008).
The gluteal tendons may be evaluated with MRI and ultrasound (in thin patients).
Patients with GTPS have peritrochanteric increased fluid signal abnormalities. However, although the absence of peritrochanteric T2 MR abnormalities makes trochanteric pain syndrome unlikely, detection of these abnormalities on MRI is a poor predictor of trochanteric pain syndrome as these findings are present in a high percentage of patients without trochanteric pain (Blankenbaker 2008). About half of the patients with GTPS present with peritrochanteric edema, and about 20 % of them (34/174) display real bursitis. The vast majority (78/91) of the patients with edema present with gluteus medius tendon degeneration, and only rarely a tear is documented (1/91). A relationship between gluteus tendinopathy and increased acetabular coverage is suggested by Klontzas and Karantanas; they calculated a significantly mean increased center edge angle (CEa) of 6° in symptomatic compared to non-symptomatic patients.
US of the greater trochanter is not easy to perform because of aliasing artifact with hypoechoic aspect of the tendons if they are not examined properly. They must be examined in both the axial and coronal planes with correct probe position (Fig. 26.12).
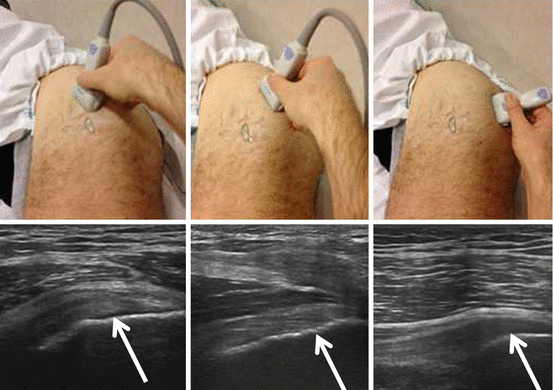

Fig. 26.12
US evaluation of the gluteal insertions on the greater trochanter. The correct probe positions are shown together with the obtained US images in the correct paracoronal planes. The image on the left shows the insertion of the gluteus minimus on the anterior facet. The image in the middle shows the insertion of the gluteus medius on the lateral facet and the image on the right the gluteus medius insertion on the posterosuperior facet. Posteroinferior facet is naked (without tendon insertion); the trochanteric bursa is located in this area deep to the gluteus maximus and distal MTJ
Tendinopathy appears ultrasonographically typically as a hypoechoic and swollen tendon. Partial tears can be seen as hypoechoic clefts (Fig. 26.13). Fiber discontinuity is demonstrated in case of tendon rupture (Fig. 26.14). US-guided treatment (infiltration of the bursae or percutaneous needle tenotomy with or without bursal infiltration) is performed in therapy-resistant tendinopathy (Klauser et al. 2013).
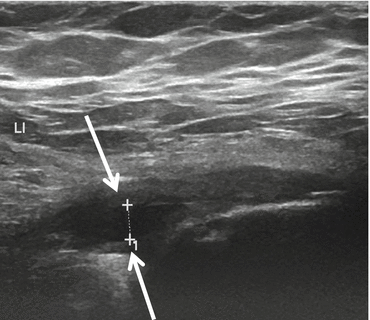
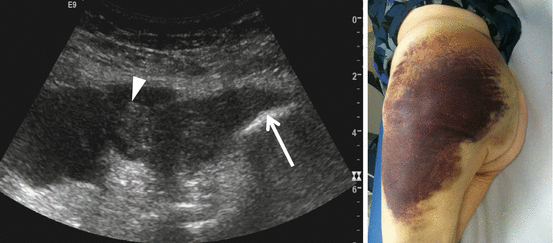

Fig. 26.13
Tear of the gluteus medius. US shows a partial tear in the insertion of the gluteus medius on the lateral facet of the greater trochanter. The tendon (arrows) is swollen and has a hypoechoic appearance and signs of tendinopathy with tendinosis. The tear is seen as the hypoechoic cleft (+ marks)

Fig. 26.14
Tear of gluteus medius and minimus. US shows rupture (arrowhead) of the gluteus medius and minimus tendons at the greater trochanter (arrow). Low-frequency abdominal probe is used to better visualize the deep structures
26.3.7 Morel-Lavallée Lesion
Morel-Lavallée lesion is a shearing injury in which the skin and the subcutaneous fatty tissue are abruptly separated from the underlying fascia (also called “closed degloving injury”).
At the hip region it is typically located at the level of the greater trochanter and is typically seen after a fall with a bike or motorcycle or skiing with shear force application on the skins. It has recently been described around the knee and can be seen on the back as well. Deglovement of subcutaneous tissues from the underlying fascia disrupts the subcutaneous vascular plexus (lymphatic and venous) at the level of the superficial fascia with leakage of serosanguinous fluid that because of the low hydrostatic pressure at the subcutaneous tissue easily persists and may result in huge soft collections. In severe cases surgery with aspiration and compression is needed. Morel-Lavallée lesion can well be evaluated with US because of its superficial localization (Choudhary and Methratta 2010; Neal et al. 2008). US can easily show the extension of the lesion (Fig. 26.15). Ultrasound-guided aspiration of the collection followed with elastic compression may be performed.
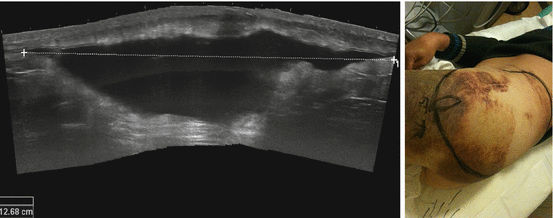

Fig. 26.15
Morel-Lavallée lesion. US shows a huge subcutaneous serosanguinous fluid collection caused by disruption of the lymph and venous plexus below the subcutaneous fat tissue after shearing injury. Image on the right shows the extension of the degloving lesion, marked with the black line and evaluated with US
26.3.8 Muscle Contusion
Next to muscle strains, traumatic muscle contusions have been reported as the most frequent type of quadriceps injury in sports. Contusions are very frequent around the hip and thigh in contact sports. Muscle contusions are a result of direct trauma, usually by a blunt object, frequently the fellow sports man’s knee in contact sports (rugby)/soccer. This causes edema, hemorrhage, and/or hematoma resulting in muscle swelling and spontaneous pain. Muscle strength typically is not reduced.
The anterior thigh is a commonly injured part of the body.
The damage with contusion usually occurs in the layer closest to the bone (intermediate vastus muscle of the quadriceps) or tendon in soccer players or pectoralis major muscle in rugby players (Hayashi et al. 2014).
Muscle contusions and strains are differentiated by the history of high stress use in the latter as opposed to the history of a direct trauma with a contusion in the former. In the case of muscle strain with incomplete tear, only minor pain is present at rest but characteristically increases with muscle contraction. Muscle ruptures are usually straightforward: sudden intense pain, tightness, and loss of function occur. The patient usually describes a popping sensation. Strain lesions usually involve the rectus femoris muscle.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree