Fig. 4.1
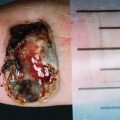
Cytology proven 8 mm hypoechoic melanoma deposit (asterisk) in a 3 cm long groin node which shows otherwise normal sonographic characteristics. Arrow indicates aspiration needle in situ
4.4 Computerised Tomography (CT)
CT is the preferred modality for assessment of melanoma metastases to lungs and abdominal organs. Pulmonary metastases from melanoma are usually multiple and may vary in size from miliary to large (Fig. 4.2) [8] and are a relatively common site of metastasis. Isolated pleural effusion is, however, uncommon [9]. Hepatic metastases are often hypervascular (Fig. 4.3) enhancing in the arterial phase and hypo- or isodense in portal venous phase, a pattern which helps distinguish them from other metastases. Metastases to the spleen, pancreas and kidneys are rare and may be solid or cystic [7] but are otherwise non-specific in appearance. Adrenal metastases may be bilateral, and metastases to the gall bladder may be small flat nodules or polypoid and pedunculate (Fig. 4.4). Metastatic lesions to the small bowel may cause wall thickening or manifest as small nodules, which may be missed without use of oral contrast but may sometimes be detected due to resultant intussusception (Fig. 4.5) or small bowel obstruction. Masses resulting from metastases to ovaries are usually difficult to distinguish from other ovarian masses on CT, and the uncommon occurrence of melanoma metastases to the vagina and uterus may be occult on CT, especially when small.





Fig. 4.2
Lung window image of CT chest demonstrating bilateral, multiple, pulmonary metastases from melanoma

Fig. 4.3
Hypervascular liver metastases. Arterial phase CT demonstrates several hypervascular hepatic metastases with varying degree of intralesional necrosis

Fig. 4.4
Portal venous phase CT demonstrates hypodense metastasis in the liver (long arrow). A metastasis in the gall bladder is identified as a polypoid mass (asterisk), and there are multiple peritoneal metastases (e.g. short arrow) in a patient with advanced melanoma

Fig. 4.5
Melanoma metastases to small bowel causing wall thickening (asterisk) and intussusception (arrow)
Metastases to the muscles may be difficult to detect especially when poorly enhancing unless they distort the contour noticeably (Fig. 4.6). Subcutaneous metastases are, however, well demonstrated even when small (Fig. 4.7). Nodal metastases especially in deep stations are better assessed with CT than ultrasound. Lesions in the breast may be difficult to detect prospectively, but can be identified as non-specific soft tissue nodule. Bony metastases and their associated soft tissue extension are well demonstrated by CT, though small lytic lesions may be occult and better detected on MRI or PET/CT (Fig. 4.8).




Fig. 4.6
Portal venous phase CT of abdomen demonstrates an intramuscular metastasis from melanoma in left external oblique muscle (arrow)

Fig. 4.7
CT chest demonstrates 8 mm subcutaneous metastases in the right anterior chest wall

Fig. 4.8
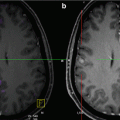
Bone window image of CT pelvis (a) in a patient with melanoma demonstrating multiple small lytic bony metastases. T1-weighted post-contrast MRI (b) done 2 days later for assessment of neuropathy shows metastases more clearly
Metastases to the brain and its acute presentation with haemorrhage are well assessed by CT, though MR is better for assessing small lesions and meningeal involvement [10]. Cerebral metastases of melanoma are usually multiple and hyperdense in pre-contrast CT and enhance following intravenous contrast (Fig. 4.9), with ring pattern of enhancement seen in 15% [11].


Fig. 4.9
Cerebral metastases from melanoma: non-contrast CT (a) shows multiple hyperdense lesions with surrounding oedema, largest in left frontal lobe with fluid level. (b and c) Right occipital and left frontal enhancing metastases (arrows in (c)), which are isodense to cortex pre-contrast (b)
4.5 MRI
MR is the imaging modality of choice for assessment of intracranial metastases from melanoma and can demonstrate even tiny metastases. Melanotic cerebral lesions are hyperintense on T1-weighted images and hypointense on T2-weighted images, whilst amelanotic lesions are hypo- or isointense to the cortex on T1 and hyper- or isointense on T2 [12]. Innate T1 hyperintensity of melanoma metastases may be from melanin or haemorrhage [10]. Micro-haemorrhage in these lesions is well depicted with gradient echo sequences or the relatively new susceptibility-weighted imaging [SWI] (Fig. 4.10). In meningeal melanoma, the leptomeninges are involved more commonly than the dura (7) leptomeningeal metastases; both intracranial and spinal are best assessed with MRI and are demonstrated as abnormal enhancement. The innate T1 hyperintensity of melanoma lesions in visceral metastasis in MRI (Fig. 4.11) can suggest the correct diagnosis but has been reported in only 10% [13].


Fig. 4.10




MR appearance of melanoma metastases to the brain: melanotic metastases in each frontal lobe are hyperintense in T1 (a), whilst an amelanotic lesion is enhancing post-contrast (arrow, b) but hypointense pre-contrast. The three lesions show varying hypointensity in SWI (c) depending on the degree of haemorrhage
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree