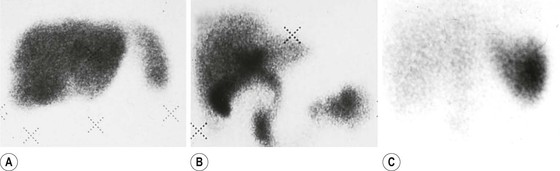
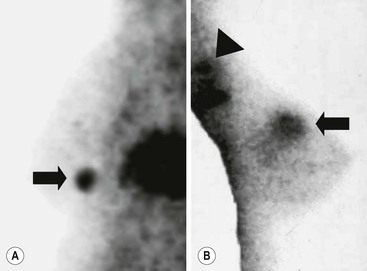
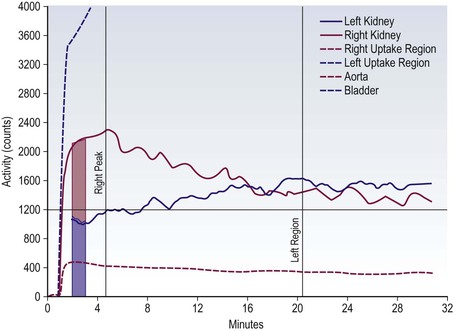
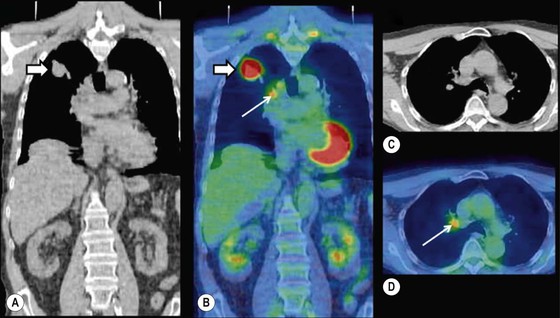
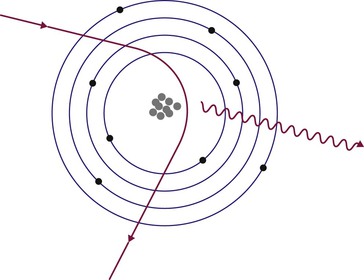
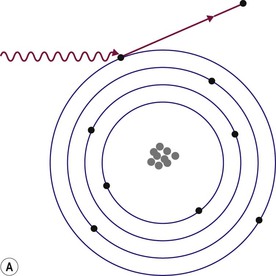
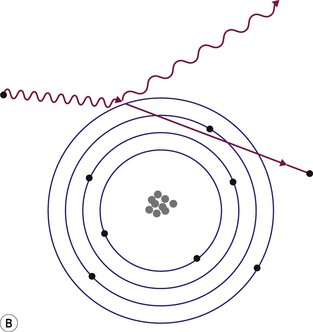
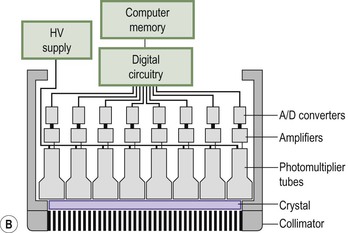
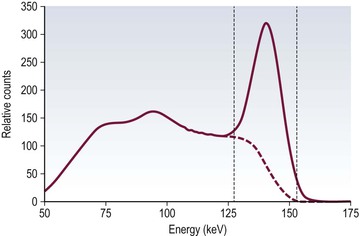
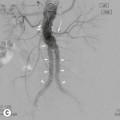
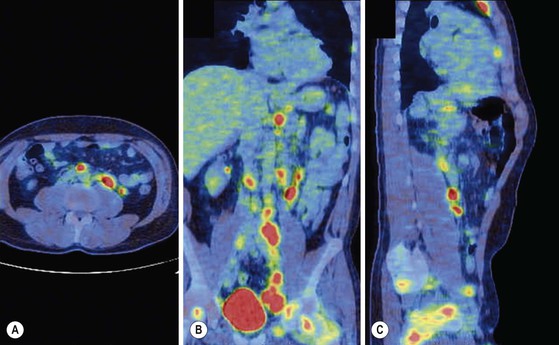
Brian F. Hutton, Djilda Segerman, Kenneth A. Miles Frequently, the first impression of radionuclide images is one of poor anatomical definition compared with other imaging techniques. If the aim of diagnostic imaging were merely the demonstration of human anatomy, the lower spatial resolution of radionuclide imaging would indeed be a disadvantage. However, the intent of diagnostic imaging is not illustration of anatomy, rather the improvement of health outcomes for patients through more accurate diagnosis. The increasing incorporation of radionuclide imaging into national clinical management guidelines illustrates radionuclide imaging’s impact on the care of patients. The development of hybrid imaging in which radionuclide imaging devices are combined with computed tomography (CT), and more recently with magnetic resonance imaging (MRI), in a single imaging system has, to some extent, addressed the limited anatomical definition of radionuclide imaging. These systems produce images in which the functional data obtained from the radionuclide distribution (usually in colour) can be overlaid on the anatomical information from CT or MRI (usually in grey scale), thereby allowing more precise anatomical localisation of areas of abnormal radionuclide accumulation (Fig. 6-1). The aim of this chapter is to describe how the ‘fuzzy’ images produced using radionuclides, with or without CT or MRI co-registration, can achieve this clinical impact. Radionuclide images depict organ function rather than structure. The images are obtained by mapping the distribution of an administered radiopharmaceutical within the body. Thus, as for X-ray radiography and computed tomography, radionuclide imaging uses ionising radiation. However, the radiation is emitted from within the patient and subsequently detected in the imaging device, rather than transmitted through the patient from an external X-ray source. The specific organ function depicted is determined by the biological behaviour of the radiopharmaceutical. For example, a radiopharmaceutical excreted by the kidney will evaluate renal function whilst one excreted in the bile will depict the biliary tree. The biological behaviour of radiopharmaceuticals can be traced to particular cellular or molecular mechanisms. Thus, by using different radiopharmaceuticals, a range of cell types can be imaged within the same organ (Fig. 6-2), or different molecular mechanisms with the same cell type (Fig. 6-3). It is perhaps ironic that, despite limited spatial resolution, radionuclide images depict structures (i.e. cells and molecules) that are too small to be visualised by anatomical imaging techniques. Another advantage of radionuclide imaging is that quantification of the amount of radiopharmaceutical within a particular organ can be achieved through counting the amount of radiation emitted within a specific time. By obtaining repeated images, the change in concentration of radiopharmaceutical over time can be measured and displayed as time–activity curves, giving additional insights into the functional status of tissues (Fig. 6-4). With appropriate mathematical modelling, these changes in concentration can be used to quantify particular physiological processes, such as glomerular filtration rate (GFR) or left ventricular ejection fraction. As repeated images can be obtained after a single administration of radiopharmaceutical, these temporal data are obtained with no additional radiation exposure to the patient. Radionuclide imaging devices are highly sensitive and can detect relatively small changes in functional activity and it is the magnitude of change in functional activity, rather than the size of an abnormality, which determines whether a disease can be detected by radionuclide imaging. Thus, a change in tissue function is frequently detectable before any change in structure has occurred or can be detected confidently by anatomical imaging. For example, increased glucose metabolism of cancer cells within lymph nodes can be detected using positron emission tomography (PET) before the size of the lymph node exceeds the threshold to be diagnosed as abnormal on CT (Fig. 6-5). Similarly, functional activity may return to normal following successful treatment before anatomical abnormalities have resolved; thus radionuclide imaging can be advantageous in monitoring treatment (e.g. in oncology). In other circumstances, the functional status of an organ is more important for determining clinical management than its structural appearance. For instance, the anatomical finding of a dilated renal collecting system does not necessarily indicate renal tract obstruction. It is the presence of obstruction and its impact on renal function, as demonstrated by renal radionuclide imaging, that more accurately determines whether clinical intervention is required. Atoms are composed of three types of particle: protons, neutrons and electrons. The protons and neutrons form a central nucleus. Each proton has a positive electric charge (+); neutrons have no charge; electrons have a negative electric charge (–) and orbit around the nucleus, attracted by its positive charge. Protons and neutrons are nearly 2000 times heavier than electrons, so most of the mass of an atom is in the nucleus. A nucleus is described by its mass number, A, which is the sum of the number of neutrons (N) and protons. The atomic number, Z, is equal to the number of protons. The mass number is therefore given by The atomic number Z is constant for a given element. The same element can have varying numbers of neutrons; these different forms of an element are called isotopes. The chemical properties of the different isotopes of an element are the same. Isotopes with more neutrons will be heavier, so some physical properties may differ. To distinguish the isotopes of an element, the mass number A is normally written as a superscript. For example, the isotope of iodine with A = 131 is written 131I. Other conventions are I-131 and 53131I. In the latter example the subscript signifies Z, the atomic number of the element. A nuclide is the term to denote a type of atom characterised by both Z and N. A radionuclide is a nuclide that is radioactive. A radioactive nucleus is one that is unstable and will spontaneously disintegrate or decay at some time. The reason for the instability is either that the nucleus is too large or because there is an imbalance between the protons and neutrons. Unstable nuclei have an excess of energy. When a nucleus disintegrates, it will emit radiation either as photons (X or gamma rays) or as high-velocity particles. The remaining protons and neutrons will form a daughter nucleus, which may itself be radioactive, or may be stable. The particular nature and energy of emissions are characteristic of each radionuclide. The radiation generally is ionising, meaning that it is highly energetic and damaging to living tissue. The rate of radioactive decay is characteristic of each radionuclide. Decay is governed by probability, which means that it is impossible to predict when a particular nucleus will disintegrate. However, with large numbers of nuclei the overall rate of decay is entirely predictable. As the nuclei in a sample of radionuclide disintegrate, the number of radioactive nuclei left is reduced, and the rate of decay reduces in proportion. This effect is described mathematically by the equation of exponential decay. An important parameter is the half-life (T1/2) which is defined as the time period during which half the nuclei will decay. For example, the half-life of 131I is 8 days: in the course of 8 days, half the 131I nuclei in a sample will have decayed, and the rate of decay will have fallen to half its original value. In a further half-life the activity will drop again by half to a quarter of the original activity (and so on). The rate of radioactive decay of a sample is called activity. The SI unit of activity is the becquerel (Bq), defined as one nuclear disintegration per second. Activity of radiolabelled compounds administered to patients for diagnostic purposes is usually measured in kBq or MBq. Originally the unit of activity was the curie (Ci), defined as 3.7 × 1010 disintegrations per second, thought to be the number of disintegrations/second obtained from 1 g of radium. Conversion factors are Table 6-1 lists some medically important radionuclides. TABLE 6-1 Physical Properties of Some Commonly Used Radionuclides Main use: g = gamma camera, n = non-imaging diagnosis, p = PET, t = therapy. Beta (Emax): maximum energy of continuous spectrum; mean energy = approximately one-third of Emax. Main source of decay data.1 Alpha decay occurs only when a heavy nucleus ejects an alpha particle. The alpha particle (α) consists of two neutrons and two protons and is identical to a helium nucleus. Alpha particles interact strongly with matter, so have a very short range, typically 1 mm or less in tissue. Within this range, alpha particles repeatedly collide with molecules, disrupting their chemistry, which is extremely damaging in living tissue. Historically, radium and radon were the principal alpha emitters of medical interest. These are no longer used in medicine. Other alpha emitters are being researched for therapeutic approaches using radiopharmaceuticals that can target the delivery of short half-life alpha emitters into cancerous cells.1 Due to their very short range, alpha particles have the potential to deliver a lethal radiation dose to small metastatic cell clusters, while mostly sparing the surrounding tissue. All work with alpha emitters must be conducted under very strictly controlled conditions. A significant health hazard would exist if these radionuclides were inadvertently ingested or inhaled, as cells lining the tracheobronchial tree or gastrointestinal tract would then receive high doses of radiation. A beta particle (beta– or β–) is an electron emitted by a nucleus. Beta decay occurs in nuclei that contain an excess of neutrons. One of the neutrons (n) will convert to a proton (p+), an electron (e–) and a neutrino (v). This can be visualised as The proton remains in the nucleus, and the beta and neutrino are emitted with high velocity. The electron and the neutrino share a set quantity of energy in variable proportions, so the electrons will have a continuous spectrum of energies. An example of beta decay is that of strontium-89 89Sr: One of the neutrons has converted to a proton, increasing the atomic number from 38 to 39; strontium is converted to yttrium. The neutrino has an extremely small mass, no electric charge and interacts extremely weakly with matter. It will have no effect on the patient. Beta particles cause damage to tissue in a similar manner to alphas, but less severely. They follow a zigzag path, undergoing many collisions with atoms. At each collision the beta gives up some of its energy, causing excitation and ionisation of the atoms. The range of betas depends on their initial energy and the electron density of the material. Typical ranges in tissue are a few millimetres; in air the range may be up to several metres. Beta emitters are routinely used to deliver therapeutic radiation doses to tumours. A positron (beta+, β+ or e+) is a particle similar to an electron except that it has a positive electric charge. The decay process is a type of beta decay, except in this case the nucleus has an excess of protons rather than an excess of neutrons: Positron emitters are used in PET imaging. An example is fluorine-18 (18F): The behaviour of positrons in tissue is very similar to beta particles, with one important difference: once a positron has been slowed down by atomic collisions, it is annihilated by interaction with an electron from a nearby atom. The combined mass of the β+ and e– is converted into two annihilation photons, each with energy 511 keV, in accordance with Einstein’s famous equation, E = mc2: The two photons are emitted at 180° to each other, a property exploited by PET. Gamma rays and X-rays are both a form of electromagnetic radiation, and have similar properties. The modern definitions are that gamma rays are emitted by an atomic nucleus in an excited state, whereas X-rays are the result of changes involving electrons. Some beta emitters produce gamma photons in the course of beta decay. The decay is a two-stage process, with beta emission leaving the nucleus in an excited state. The excited nucleus then decays to its stable daughter, emitting the excess energy as a gamma photon. There may be several possible excited states, each with a particular probability of occurrence. The result will be a range of gamma photons, each with its specific energy and probability. An example is the beta emitter 131I, used primarily for therapy, but whose principal gamma ray is used in imaging. Certain excited nuclei do not decay immediately but remain in their excited state for some time. These nuclei are termed metastable. This type of decay is known as an isomeric transition (IT) because the parent and daughter nuclei are isomers; i.e. they contain the same number of protons and nuclei. An example is the metastable radionuclide of technetium, 99mTc, which emits a single gamma photon of energy 140 keV, and is the most commonly used radionuclide for imaging. In many decay schemes, nuclear energy is transferred to the atom’s orbital electrons. When the orbital electrons revert to their ground state they emit characteristic X-rays. In some cases, instead of producing an X-ray, the energy will eject an outer orbital electron as an Auger electron, with properties similar to a beta particle. Electron capture (EC) is another radioactive decay process that can occur in a nucleus with excess protons. One of the orbital electrons is captured by a proton in the nucleus and changes the proton into a neutron. If the original nucleus has enough excess energy to produce two 511-keV photons, it may decay by either positron emission or electron capture. If the excess energy is less than 1022 keV, then positron emission cannot occur and electron capture is the only decay mode. Electron capture can be visualised as Some of the energy is carried off by the neutrino and some is transferred to the orbital electrons of the atom, resulting in characteristic X-rays and possibly Auger electrons. In some cases EC will leave the nucleus in an excited state, which will decay to its stable daughter by gamma emission. Beta particles and Auger electrons can give rise to another form of radiation called bremsstrahlung (German for braking radiation). When a high-energy beta particle passes close to the nucleus of another atom, electrostatic attraction deflects and decelerates the beta particle, and its lost energy is converted to an X-ray photon (Fig. 6-6). This bremsstrahlung radiation has a continuous spectrum. The energy tends to be higher when the betas have high energy and the material they are passing through has a high atomic number. For this reason radiation shields for high-energy beta emitters are made of plastic which has a low atomic number. Two types of interaction are important in nuclear medicine (Fig. 6-7): In both interactions an orbital electron is ejected. It will lose its energy locally in the same way as a beta particle. The Compton-scattered photon will subsequently undergo either another Compton scattering or photoelectric absorption. Unlike alphas and betas, gamma rays and X-rays do not have definite ranges but are attenuated on passing through matter. A certain fraction of the photons will pass through, the fraction depending on the material, its thickness and the energy of the photons. The stopping power of a particular material is defined by its linear attenuation coefficient µ and the amount of attenuation experienced by photons travelling through tissue is expressed as where C is the counts recorded after travelling a distance d through materials with attenuation coefficient µ, and C0 is the counts that would be detected in air. The main components of the gamma camera (Fig. 6-8), originally developed by Anger, are a scintillation detector coupled to a set of photomultipler tubes (PMTs).2 In front of the detector is a collimator, which limits detectable photons to those travelling ideally at right angles to the detector; without this the origin of the photons would be unknown. The crystal converts the incoming gamma ray to light, which is detected by the array of PMTs, which in turn convert the light to electrical signals. These signals are processed to determine the location on the detector and the energy of each detected photon; the photons of energy within a selected energy window are summed to form the image, which is stored on computer. Depending on the clinical information required, this computer may, for example, track changes with time, or utilise the data to reconstruct a tomographic set of slices similar to CT or MRI. A more detailed description follows. The scintillator is usually a single crystal of sodium iodide doped with thallium (NaI(Tl)). It is typically large and rectangular, perhaps 40 × 55 cm, and usually 9.5 mm thick. When a gamma photon enters the crystal, electrons are ejected from their atomic orbits in photoelectric or Compton interactions (see above). The ejected electrons collide with nearby atoms in the crystal and energise them. The atoms of the crystal release their excess energy by scintillation, giving off a characteristic blue light. In most cases all the energy of the incoming gamma photon is converted to scintillation light. Attached to the back of the crystal is an array of up to 90 PMTs. These extremely sensitive light detectors are coupled to the crystal with special grease to give maximum transmission of light from the crystal. Each PMT consists of an evacuated glass envelope, containing a series of electrodes. A scintillation photon striking the first electrode causes emission of an electron. This electron is accelerated towards the next electrode, which emits two or three electrons for every one striking it. This amplification process is repeated at each electrode in the cascade, until at the last electrode the signal may be amplified by a factor of about 1 million. Image formation is accomplished by combining the signals from several PMTs. In the crystal, the scintillation light resulting from one gamma photon is given off in all directions, and is picked up by the nearby PMTs. From the relative size of signals from all the PMTs, the detector determines the position of the gamma photon’s interaction in the crystal to within a few millimetres, even though each PMT is several centimetres in diameter. It is important to exclude from the image radiation that has been scattered within the patient before interacting with the crystal. Ideally only those gamma rays that trace back to the distribution of radioactive material in the patient are used for image formation. Exclusion of scattered photons is made possible because the scattered rays have reduced energy (see above, Compton scattering). Figure 6-9 shows a typical energy spectrum for 99mTc displaying the number of events detected by the gamma camera across a range of photon energies. The tall peak, known as the photopeak, represents mainly unscattered gammas. These are recorded with an energy close to their original energy, which is 140 keV in the case of 99mTc (the spread around 140 keV is due to limited energy resolution in the detector: ~10% for NaI(Tl). The scattered gammas appear as the broad distribution to the left of the photopeak. The detector identifies the energy of each incoming gamma photon by summing the signals from all the PMTs. A suitable energy window is set around the photopeak as shown in Fig. 6-9. Each gamma within the energy window will register one count, and is saved in the pixel corresponding to the X and Y coordinates that denote the position of the photon interaction within the crystal. As most of the scattered gammas have energies outside the window, they are excluded from the image.
Radionuclide and Hybrid Imaging
Introduction and General Principles
Basic Physics
Structure of the Atom
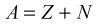
Radioactivity
Radionuclide
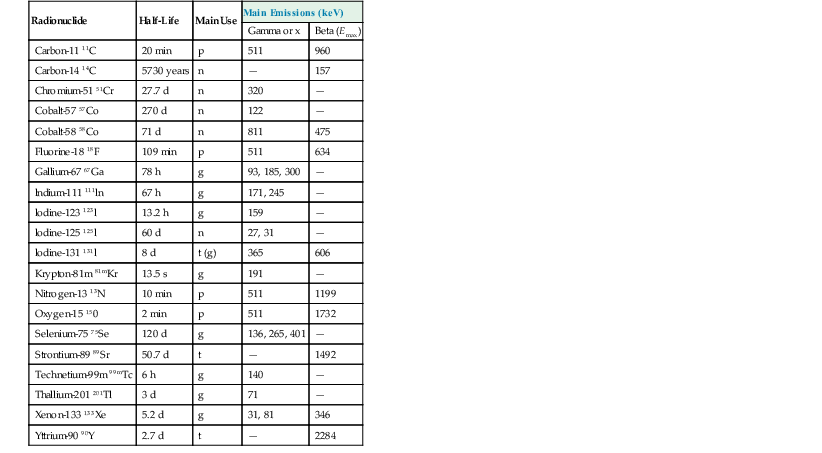
Half-Life
Main Use
Main Emissions (keV)
Gamma or x
Beta (Emax)
Carbon-11 11C
20 min
p
511
960
Carbon-14 14C
5730 years
n
—
157
Chromium-51 51Cr
27.7 d
n
320
—
Cobalt-57 57Co
270 d
n
122
—
Cobalt-58 58Co
71 d
n
811
475
Fluorine-18 18F
109 min
p
511
634
Gallium-67 67Ga
78 h
g
93, 185, 300
—
lndium-111 111ln
67 h
g
171, 245
—
lodine-123 123l
13.2 h
g
159
—
lodine-125 125l
60 d
n
27, 31
—
lodine-131 131l
8 d
t (g)
365
606
Krypton-81m 81mKr
13.5 s
g
191
—
Nitrogen-13 13N
10 min
p
511
1199
Oxygen-15 150
2 min
p
511
1732
Selenium-75 75Se
120 d
g
136, 265, 401
—
Strontium-89 89Sr
50.7 d
t
—
1492
Technetium-99m 99mTc
6 h
g
140
—
Thallium-201 201Tl
3 d
g
71
—
Xenon-133 133Xe
5.2 d
g
31, 81
346
Yttrium-90 90Y
2.7 d
t
—
2284
Alpha Radiation
Beta Radiation
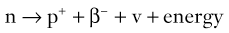
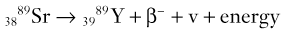
Positron Emission
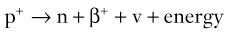
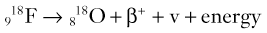
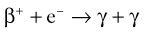
X and Gamma Radiation
Definitions
Reactions that Produce X and Gamma Radiation
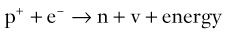
Interaction of X and Gamma Radiation with Matter
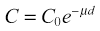
The Gamma Camera
Detection System
Radionuclide and Hybrid Imaging
Chapter 6