Radionuclide Pulmonary Imaging
M. Reza Habibian
Chirayu Shah
Clinical Indication
Ventilation/Perfusion Scintigraphy for Diagnosis of Pulmonary Emboli
Despite the increasing use of spiral CT angiography, the ventilation/perfusion lung scan continues to play an important role as a noninvasive procedure for the diagnosis of pulmonary emboli (1).
Pulmonary Imaging for Non-embolic Disease
Perfusion lung scan continues to be the functional image used for the estimation of regional pulmonary perfusion in order to predict postoperative pulmonary function after lobectomy or pneumonectomy (2). It is also useful in identifying the target area for resection in surgery for lung volume reduction and provides modest prognostic information (3).
The use of gallium-67 (67Ga) scanning for the evaluation of pulmonary disease has diminished significantly in favor of positron emission tomography (PET). However, the gallium scan is useful in the evaluation of pulmonary sarcoidosis, HIV-positive patients with suspected Pneumocystis carinii pneumonia, drug- and radiation-induced pneumonitis, lung diseases associated with pneumoconiosis, and in monitoring response to therapy in patients with lymphoma (4).
PET is a noninvasive imaging modality that can differentiate benign from malignant nodules and thus replace some of the more invasive procedures. PET imaging with fluoride 18 (18F) FDG is used more frequently for the characterization of solitary pulmonary nodules.
Ventilation/Perfusion Scintigraphy for Diagnosis of Pulmonary EMBOLI
As a result of the development of advanced multislice spiral CT technology as well as increased availability and acceptance of pulmonary CT angiography, the role of ventilation/perfusion lung scanning in the diagnosis of pulmonary emboli (PE) must be redefined (5,6).
If the chest radiograph is normal and there is no history of significant cardiopulmonary disease, the V/Q lung scan is an effective noninvasive initial study for the diagnosis of acute PE. A normal scan excludes PE and a high-probability scan can make the diagnosis.
When the chest x-ray finding makes it likely that the lung scan will not provide a clear diagnosis, CT angiography appears to be the first choice for many clinicians. The PIOPED II investigators also prefer CT angiography over V/Q scanning for diagnosing pulmonary embolism in many situations. However, a negative pulmonary CT angiogram does not have the same negative predictive value as a normal V/Q scan (1).
Methodology
Perfusion Scan
Perfusion lung scintigraphy is performed after the intravenous injection of technetium 99m (99mTc) macroaggregated albumin (MAA) with the patient in the supine position to avoid preferential lower lobe distribution. The injected particles are distributed in the lung in proportion to the regional pulmonary perfusion and are trapped in the pulmonary capillary and precapillary arterial beds. The usual dose of 3 to 5 mCi contains approximately 30,00 particles approximately 30 μm in size (10 to 90 μm). Blocking 1 out of 1,00 of a total 30 million precapillary arterial beds leaves a wide margin of safety, and no adverse hemodynamic effect is to be expected. However, in patients with severe pulmonary arterial hypertension as well as known or suspected right-to-left shunt, the number of particles should be reduced. A diagnostic scan still can be obtained using as few as 70,00 to 10,00 particles.
After entrapment in the pulmonary vascular bed, the particles undergo degradation and clear from the lung with a biologic half-time of 3 to 12 hours, eventually being phagocytized by the hepatic Kupffer cells. Demonstration of minimal activity in the liver on the day after lung scan should not be considered abnormal, but activity seen in other tissues, such as kidneys or brain, may signify the presence of an intra- or extracardiac shunt.
Pregnancy is a relative contraindication for radionuclide imaging, including pulmonary scintigraphy. The total dose and its appropriate number of particles should be kept to a minimum that still provides diagnostic information. A dose of 1 to 2 mCi containing 10,00 particles can be used for diagnostic purposes. However, from the standpoint of risk-to-benefit analysis, one should not be deterred from performing the scan after obtaining written informed consent. Interruption of breast-feeding following a perfusion lung scan is debatable but may not be necessary with the low dose of 1 to 2 mCi.
Ventilation Scan
The addition of ventilation images permits the determination of the regional airway abnormality and improves the specificity of a lung scan for diagnosis of PE. A positive predictive value of a perfusion scan as compared with combined V/Q images can be improved from 59% to 92% excluding a significant number of nondiagnostic scans.
Of the current radiopharmaceuticals available for ventilation scanning, xenon 133 (133Xe) and 99mTc diethylenetriamine pentaacetic acid (DTPA) aerosol are the two most commonly used.
133Xe is a radioactive gas with a half-life of 5.3 days. It decays by beta and gamma radiation. The photon energy is 81 keV.
The 133Xe ventilation scan consists of three consecutive phases of single breath holding, equilibrium, and a washout phase. The washout phase is the most sensitive phase of the ventilation scan for the detection of airway disease.
Despite its sensitivity in detecting airway disease, 133Xe is not an ideal agent. There are several disadvantages to its use: notably poor spatial resolution of the image due to its
low-energy photon, relatively high radiation dose to the lung, and complexity of its disposal. Another significant disadvantage is the inability to acquire images in multiple projections.
low-energy photon, relatively high radiation dose to the lung, and complexity of its disposal. Another significant disadvantage is the inability to acquire images in multiple projections.
As an alternative to 133Xe, a diagnostically equal scan can be obtained with radioaerosol inhalation, which also has the advantage of providing images in multiple projections. This is very helpful for direct comparison with the perfusion images that best identify the defect.
The radioaerosol in current use is 99mTc-DTPA. A dose of approximately 30 to 40 mCi of 99mTc-DTPA is introduced into the commercially available nebulizer, which generates submicronic particles ranging from 0.1 to 0.5 μm. Through the closed system, the patient breathes a mixture of oxygen and radioaerosol for 3 to 5 minutes. This process deposits the radioaerosol into the bronchoalveolar space, thus mapping the distribution of ventilation.
Approximately 500 to 700 μCi of radioactivity reaches the lung, which is sufficient to obtain multiple images within a few minutes. The ventilation scan is usually obtained before the perfusion scan. Residual 99mTc activity from the ventilation scan will be easily overridden by a higher dose of 99mTc macroaggregated albumin (MAA) used for perfusion images.
The distribution of the aerosolized particles is affected not only by particle size but also by the presence of airway disease, which results in excessive central deposition due to turbulent airflow and poor alveolar penetration of inhaled particles. This may decrease the quality of the image, thus lowering its diagnostic validity.
Once deposited in the lungs, the 99mTc-DTPA clears from the lung across the alveolar capillary membrane into the bloodstream, with a biologic half-time of 1 to 1.5 hours; it is excreted by the kidneys. Significantly increased renal activity after ventilation scan may signify an epithelial injury state, such as damage from cigarette smoking or interstitial pulmonary fibrosis (Fig. 2.A).
Imaging Technique
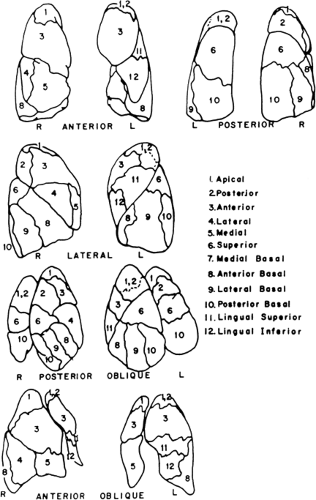
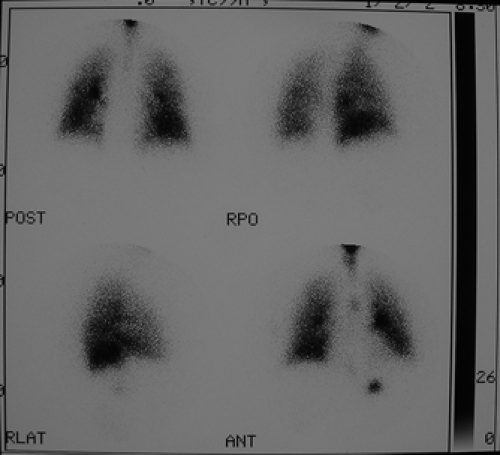
Standard projections for both aerosol and perfusion scans are anterior, posterior, right posterior oblique (RPO), left posterior oblique (LPO), right lateral, left lateral, and preferably right anterior oblique (RAO) and left anterior oblique (LAO).
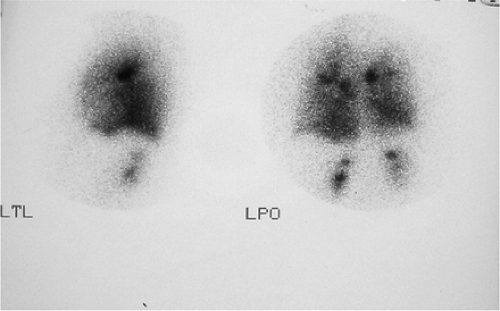 Figure 2.A 99mTc DTPA ventilation scan of a patient with interstitial pulmonary fibrosis showing increased renal activity. |
After the completion of ventilation imaging, the patient is injected for perfusion scan. Injection is done in the supine position, which allows more uniform distribution of blood flow to the upper and lower lung fields. Images of perfusion are obtained in the same order, same projection, and same patient position as for ventilation, preferably with the patient in a sitting position. This reduces the ambiguity and differentiation in scan appearance due to different position and variability related to the position of the heart, diaphragm, and mediastinum.
Lateral and oblique views are important projections that only may fully demonstrate the segmental nature of the perfusion defect. The lateral segments of the lower lobe are better appreciated in the posterior oblique projection, as the lingula and middle lobe are best seen in anterior oblique projection. Oblique views also have the advantage of avoiding shine-through activity, which degrades the quality of lateral projections. The contribution of shine-through activity could be as high as 30%.
Normal Scan
The normal aerosol inhalation scan shows a uniform distribution of radioactivity outlining the lung fields. Minimal deposits of aerosol may be seen in the central airway (airway hot spot) as well as in the stomach and bowel, representing swallowed radioactive aerosol.
The normal perfusion scan also shows uniform distribution of radioactivity outlining the lung fields. The silhouette of the heart, mediastinum, hilum, aortic knob, diaphragm, and spine are readily identifiable.
Variation from normal in the pulmonary perfusion pattern is not common. Segmental defects rarely represent a normal variation; however, the presence of a subsegmental, subapical defect is not uncommon among normal subjects. To avoid overcall of the perfusion defect, attention should be given to the chest wall configuration as well as technical details during imaging. A chest wall deformity such as scoliosis or pectus excavatum may cause an apparent defect. Attenuation caused by interposition of the patient’s arm on the lateral projection should not be confused as a defect. In the majority of these patients, the defect disappears by changing the patient’s position slightly.
Interpretation of Ventilation/Perfusion Lung Scans
Several schemes for interpretation of ventilation-perfusion scintigram have been developed over the years. These included the Bilo, original PIOPED, and the revised PIOPED (Table 2.1) criteria. Currently, another set of categories has been suggested by the PIOPED II study. PIOPED II is a prospective multicenter study designed to objectively evaluate the new scan categories to include a very low probability category and also to evaluate the efficacy of CT in the diagnosis of PE.
In PIOPED II, the revised PIOPED criteria for high probability have been maintained, the intermediate and low probabilities have been revised, and a very low probability criterion has been established (7,8).
TABLE 2.1 Revised PIOPED criteria for V/Q scan interpretation | |||||
|---|---|---|---|---|---|
|
The very low probability criteria in PIOPED II are as follows:*
Nonsegmental perfusion abnormalities (enlargement of the heart or hilum, elevated hemidiaphragm, linear atelectasis, or costophrenic angle effusion with no other perfusion defect in either lung).
Perfusion defect smaller than corresponding radiographic lesion.
≥2 matched V/Q defects with regionally normal chest radiograph and some areas of normal perfusion elsewhere in the lungs.
1 to 3 small segmental perfusion defects (<25% of a segment).
Solitary triple matched defect (defined as a matched V/Q defect with associated matching chest radiographic opacification) in the middle or upper lung zone confined to a single segment.
Stripe sign, which consists of a stripe of perfused lung tissue between a perfusion defect and the adjacent pleural surface (best seen on a tangential view).
Pleural effusion equal to one third or more of the pleural cavity with no other perfusion defect in either lung.
A very low probability interpretation, according to these authors, has a positive predictive value (PPV) of 8.2%. However, a very low probability scan combined with a low clinical probability has a PPV of 3.1%, and when these data were applied only to a female patient aged <40 years, it was only 2%. Based on these findings, the authors conclude the combination of a low clinical probability and very low probability interpretation reliably excludes acute pulmonary embolus and can be used in patients whose computed tomography angiography (CTA) may be disadvantageous (women of reproductive age, compromised renal function, allergic risk).
Regardless of what criteria are being used, the classification of lung scans as normal, high probability, or very low probability is easier and the results are more reliable. However, distinguishing between low probability and intermediate probability may be difficult and some degree of uncertainty is unavoidable. In addition, an interobserver variability as much as 30% is not uncommon. Therefore adhering to the strict diagnostic criteria of lung scan interpretation may not be practical for all patients all the time. It appears that using the criteria combined with interpretative experience (gestalt), clinical evaluation, and consideration of the total situation is the best approach for the diagnosis of PE. The combination of clinical assessment and V/Q interpretation improves the chances of making the correct diagnosis when compared with either scan or clinical assessment alone (Table 2.2). It is important to note that the scintigraphic findings and clinical impression of PE are more commonly concordant than discordant.
The basic concept in formulating a rational approach to the diagnosis of PE is to observe all the clinical data—pretest probability factors and stratification of the patient with respect to underlying cardiopulmonary disease as well as ancillary scintigraphic finding and radiographs that either favor or disfavor the presence of embolic disease (9).
Consideration should be given to the conditions that affect the clinical probability of PE. Objective clinical probability is assessed according to the Wells test and are as follows†:
TABLE 2.2 Percent probability of acute pulmonary embolus using combinations of clinical and scan findings
Clinical probability
Scan probability
High
Intermediate
Low
Normal
High
96
66
40
0
Intermediate
84
25
12
4
Low
56
16
4
2
Clinical signs and symptoms of deep venous thrombosis (DVT) (3 points)
Heart rate >100 beats/minute (1.5 points)
Immobilization ≥3 consecutive days or surgery in previous 4 weeks (1.5 points)
Previous objectively diagnosed PE or DVT (1.5 points)
Hemoptysis (1 point)
Malignancy (cancer patient receiving treatment within 6 months or receiving palliative treatment) (1 point)
PE as likely as or more likely than alternative diagnosis (based on history, physical examination, chest radiograph, ECG and blood test) (3 points)
A score of less than 2 considered as clinically low probability.
A score of 2 to 6 is intermediate, and a score of greater than 6 accounts for high clinical probability.
Although the history of DVT constitutes a moderate clinical risk for pulmonary emboli (20% to 70%), the presence of concurrent DVT is a high risk (70% to 80%) factor. Sonographic evidence of DVT may be seen in up to 30% of patients who have a PE and only in 15% of patients with an intermediate probability scan. Therefore a negative Doppler examination, as seen in 85% of intermediate scans, should not deter attention from the diagnosis of PE. Negative Doppler examinations in the majority of patients with angiographically confirmed PE do suggest that the thrombus has already migrated to the lung.
Pulmonary emboli tend to be multiple, thus producing more than two perfusion defects accompanied by a normal ventilation scan and clear chest x-ray. The wedge defect that occupies the lower and lateral border of the lower lobe seen in the anterior and lateral projections represents impaired perfusion of the two nearby segments (anterior and lateral-basilar segments). The perfusion defect of pulmonary embolism is wedge-shaped and pleural-based; therefore a centrally located defect is evidence against PE. The segmental nature of perfusion defect is also an important characteristic of PE. Bronchopulmonary segments of the lung have a rather defined boundary that can be appreciated on the surface map of the lung (Fig. 2.B). Defects that are irregular in shape and do not respect segmental boundaries are unlikely to be due to PE.
Perfusion defect along the fissures (fissure sign), although originally thought to be due to microembolism along the fissure, is usually related to the pleural effusion in the fissure or is caused by chronic lung disease and is not in favor of PE (Fig. 2.C).
Unilateral absence or near absence of perfusion in association with ventilatory abnormality strongly suggests airway disease and is not in favor of PE. In addition to the malignant and benign bronchogenic mass, stenosis of the pulmonary artery, or presence of a foreign body must be considered.
Multiple small serrated defects along the contour of the lung, known as a contour pattern, are usually associated with tumor microembolization and lymphatic spread of carcinoma and should not be mistaken for pulmonary embolism secondary to thrombus.
Although 75% of perfusion defects resolve within 3 months, the remaining 25% of defects may persist for years and thus lead to a false-positive interpretation lung scan. In patients
with documented previous PE, the positive predictive value of a high-probability scan is lower (74%) than in those without previous PE (96%). Comparison with a prior scan in such a patient is extremely important.
Role of the V/Q Scan and CT Angiogram in the Management of Patients With Suspected PE
As mentioned earlier, the PIOPED II clinical trial investigators prefer CT pulmonary angiography over ventilation perfusion lung scanning for diagnosing PE in many situations (10). However, pulmonary CT angiography will be more diagnostic if it is combined with CT venography of the popliteal and femoral veins and the overall result are evaluated in the proper context of pretest probability. In absence of clinical assessment and CT venography, the pulmonary CT angiogram has a sensitivity of only 83%, which is not adequate for the management of PE.
Considering the risk of iodinated contrast, particularly in patients with impaired renal function, and the risk of radiation to the breast in women of reproductive age, pulmonary CT angiography may not be the first-line test for the diagnosis of PE in these patients. Alternatives such as the combination of venous ultrasound and V/Q lung scan may be more appropriate, as the use of the V/Q scan minimizes radiation to the breast.
One of the recommended approaches in the selection of V/Q scanning versus pulmonary CT angiography for providing the most favorable outcome (considering cost, risks, and availability) is based on the pretest probability of PE (11).
In patients with high pretest probability, the presence of PE can be confirmed by either V/Q scanning or CT angiography. Both have the same discriminatory power in confirming PE.
In patients with low pretest probability, PE can be excluded by CT angiography; if this is not available, however, a normal or near normal V/Q scan is an alternative.
In excluding PE in patients with moderate pretest probability or in confirming PE in patients with low pretest probability, the V/Q scan should be avoided, as the CT angiogram has higher discriminatory power for these categories.
Case 2.1
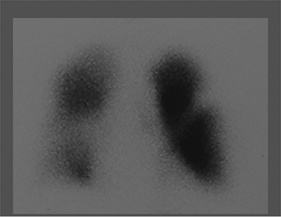
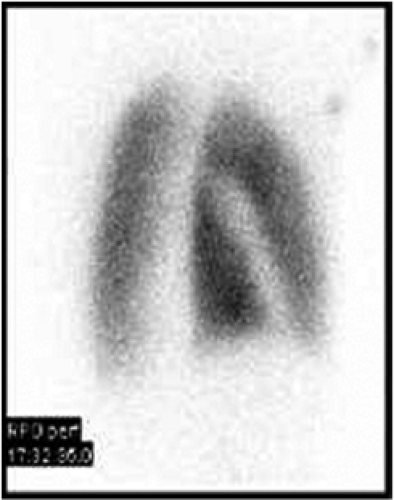
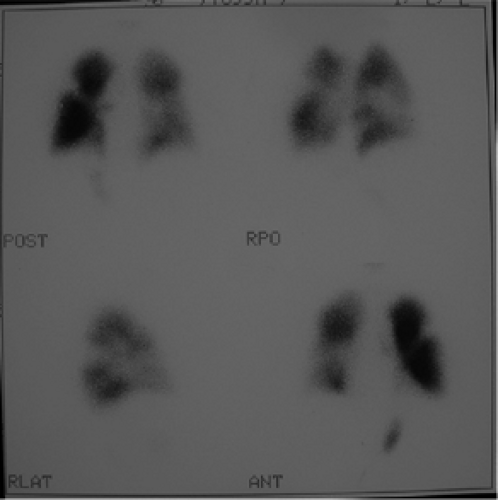
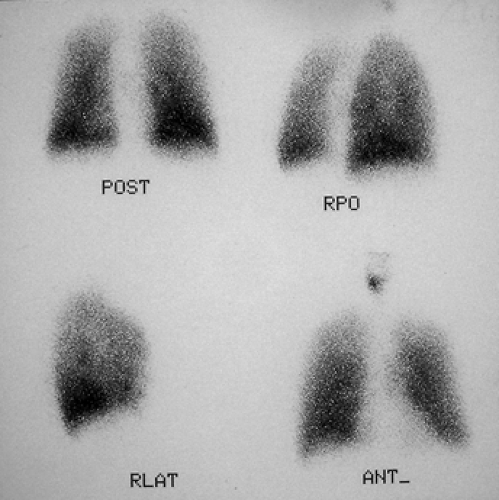
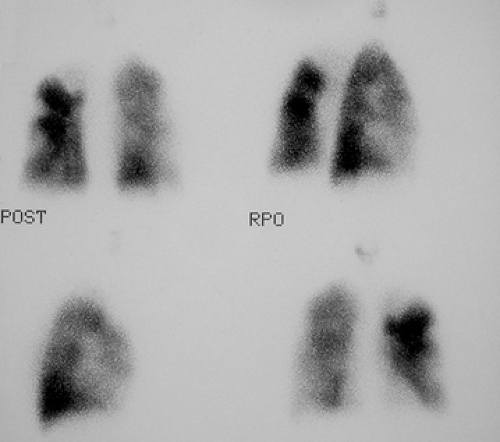
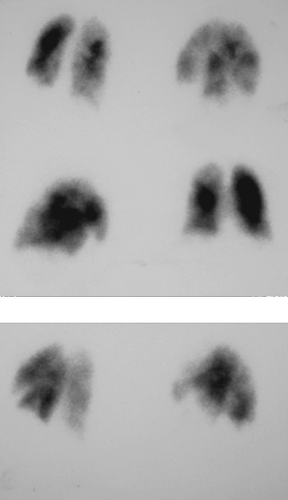
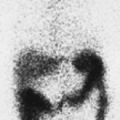
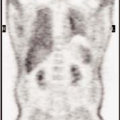
History: A healthy 36-year-old male with a family history of thrombophilia (not known at the time) presents with chest pain, dyspnea, and apprehension. His chest x-ray is normal. He receives a V/Q scan for evaluation of probable pulmonary emboli (Figs. 2.1 A, B).
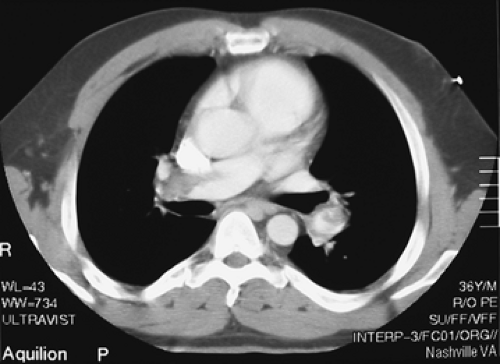
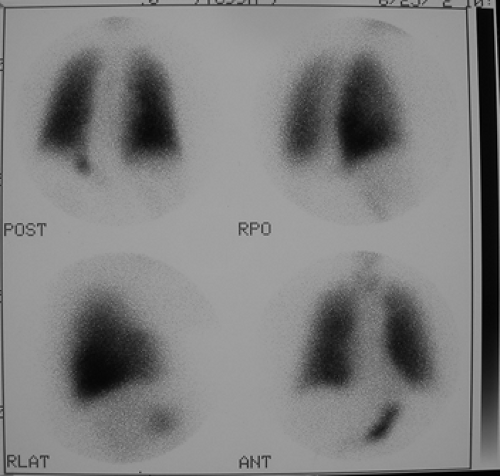
Findings: There are multiple large, wedge-shaped, pleural-based, mismatched defects in both lung fields. In addition, there is generalized reduced perfusion of the right lung as compared with the left. The lung scan findings indicate a high probability of acute pulmonary emboli, which were also visualized on CT angiography (Fig. 2.1 C) done on the same day. Multiple filling defects within the lumen of contrast-filled pulmonary arteries are seen on both sides. A follow-up perfusion scan (Fig. 2.1 D) after completion of anticoagulation therapy shows complete resolution of the perfusion abnormalities.
Discussion: Multiple large, segmental, mismatched perfusion defects are important characteristics for pulmonary emboli which, in patients with high clinical probability, have a positive predictive value of 96%.
Other causes of V/Q mismatch are previous PE, bronchogenic carcinoma, previous radiation therapy to the lung, pneumonia, tuberculosis, collagen vascular disease, intravenous drug abuse, tumor emboli, etc.; however, most of these can be excluded by clinical and other diagnostic tests.
Perfusion defects due to acute pulmonary emboli gradually regress with time and following therapy; the majority resolve within 3 months. Resolution is quicker in patients with no previous cardiovascular disease (3 to 7 days).
Obtaining a perfusion lung scan at the completion of anticoagulant therapy and at discharge is of clinical value in future management of the patient. This scan will serve as a baseline for the diagnosis of recurrent PE, which is seen as the development of a new defect.
Most patients with high-probability lung scans and a high clinical likelihood of PE require treatment and need no further diagnostic test to confirm diagnosis. Utilization of CT angiography in the workup of patients suspicious for PE is increasing. However, in patients with a high probability for PE, the discriminatory power of V/Q and CT angiography is similar and concordant positive results have been reported in 86% of interpretations.
In evaluating patients with PE, attention should be given to the risk factors for the development of DVT and PE, the most notable of which are use of estrogen, history of previous DVT or PE, and presence of concurrent DVT. Genetic disorder of thrombus formation (thrombophilia) should be kept in mind when PE occurs in young patients with no other predisposing factors. These patients are characteristically younger than the average patient with thrombosis.
Diagnosis: Acute pulmonary embolism with complete resolution.
Case 2.2
History: A 55-year-old male who had undergone renal transplantation and now has declining renal function was flown from a long distance to the Medical Center for further evaluation and possible renal biopsy.
Shortly following his admission, the patient developed a sudden onset of right-sided chest pain and dyspnea. Cardiac enzyme, ECG, and chest radiographs were not contributory and the patient received a V/Q scan for the evaluation of probable pulmonary emboli.
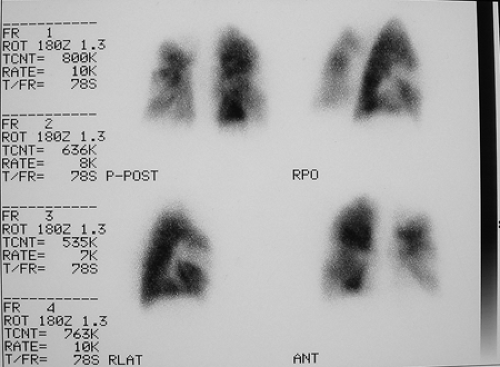
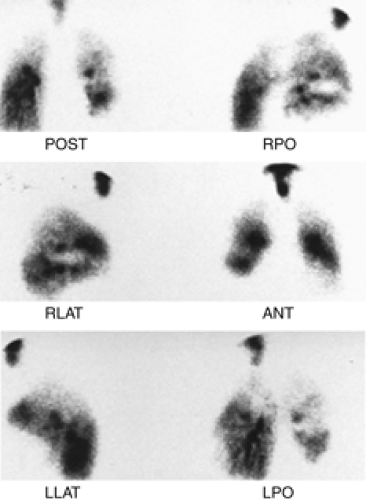
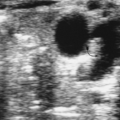
Findings: V/Q lung scans (Figs. 2.2 A, B) show multiple large and moderate sized wedge-shaped, pleural-based, mismatched defects in both lungs.
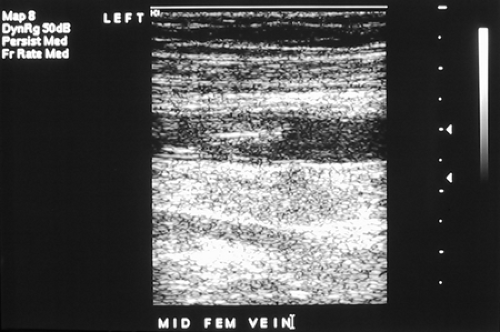
Immediately following the lung scan, a lower extremity venous Doppler examination (Fig. 2.2 C) was performed. The duplex study shows large segmental venous thromboses involving the midportion of the superficial femoral vein and extending through the left popliteal vein.
The patient was managed appropriately for thromboembolic disease. A lung scan at 3-month follow-up (Fig. 2.2 D) shows persistence of multiple defects with no significant interval change.
Discussion: In the proper clinical context and in a patient with no history of previous pulmonary emboli and a clear chest radiograph, the presence of multiple large mismatched segmental defects represents a high-probability study with a positive predictive value of 96%.
In the diagnosis of PE, regardless of the scan category, attention should be paid to the clinical presentation as well as risk factors contributing to the formation of DVT and subsequent PE. Genetic factors, use of oral contraceptives, pregnancy, puerperium, malignancy, immobilization, trauma, recent surgery, central line placement, and long airplane flight (traveler’s thrombosis) are among the most important risk factors to consider.
Coexistence of PE and DVT is not unusual. The majority of PE arise from the proximal vein of the lower extremity. About one-third of patients who have PE also have detectable DVT, but more than half of the patients with PE do not demonstrate thrombosis, suggesting that the thrombus has already migrated to the lung.
Silent emboli are also not uncommon and suspected perfusion defects consistent with PE may be seen in up to 30% of patients with a positive venous duplex scan. Although these patients normally receive anticoagulant therapy, obtaining a baseline perfusion lung scan would be helpful for evaluation of future episodes of PE.
Perfusion defects resulting from pulmonary emboli may begin to resolve within 3 to 4 days. Resolution of perfusion defect is quicker in patients with no previous cardiopulmonary disease but slower when a large defect occurs in a patient with preexisting cardiopulmonary disease. A defect not resolving by 2 to 3 months may persist for many years, and some of these patients may develop chronic changes of pulmonary emboli. It is to be noted that the most common cause of a false-positive high-probability interpretation of a lung scan is the history of PE in the past.
Pulmonary emboli are responsible for a substantial number of patients suffering from a secondary pulmonary arterial hypertension. The process is initiated by single or recurrent episodes of PE. The unresolved thrombosis proceeds to the organization stage and fibrosis, which results in stenosis or complete obstruction of the pulmonary artery; this is demonstrable by CT or conventional pulmonary angiography. On lung scan, these patients would have segmental defects in both lung fields that remain unchanged over time. These patients may benefit from the surgical procedure of pulmonary endarterectomy. It is to be noted that although CT angiography appears to be
the first-line imaging procedure for the evaluation of patients suspected of having PE, V/Q pulmonary scintigraphy has a higher sensitivity and specificity for distinguishing pulmonary arterial hypertension of chronic thromboembolic disease from other causes of pulmonary arterial hypertension (idiopathic, emphysema, pulmonary fibrosis, pulmonary venous occlusive disease, sarcoidosis, and congenital heart disease such as atrial septal defect). A normal scan practically excludes chronic PE as the cause of the patient’s pulmonary arterial hypertension. The patient with a chronic inflammatory disorder of the lungs and those with splenectomy are at especially high risk for developing chronic embolic pulmonary arterial hypertension.
the first-line imaging procedure for the evaluation of patients suspected of having PE, V/Q pulmonary scintigraphy has a higher sensitivity and specificity for distinguishing pulmonary arterial hypertension of chronic thromboembolic disease from other causes of pulmonary arterial hypertension (idiopathic, emphysema, pulmonary fibrosis, pulmonary venous occlusive disease, sarcoidosis, and congenital heart disease such as atrial septal defect). A normal scan practically excludes chronic PE as the cause of the patient’s pulmonary arterial hypertension. The patient with a chronic inflammatory disorder of the lungs and those with splenectomy are at especially high risk for developing chronic embolic pulmonary arterial hypertension.
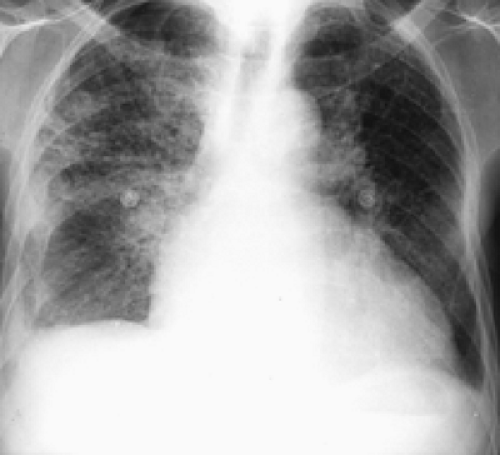
Figures 2.2 E and 2.2 F shows a chest radiograph and perfusion scan of a 60-year-old patient with pulmonary arterial hypertension secondary to PE. On perfusion scan (Fig. 2.2 F), in addition to the presence of a segmental perfusion defect, there is generalized reduced perfusion of the right lung as compared with the left, correlating with increased lucency and reduced vascularity of the right lung seen on the radiograph (Fig. 2.2 E); this suggests a previous embolus in the right pulmonary artery. Reduced perfusion is more pronounced peripherally, consistent with diminished peripheral flow due to attenuation and pruning of the peripheral vascular branches, a known feature of pulmonary arterial hypertension.
A patient with primary pulmonary arterial hypertension may present with clinical and radiographic findings similar to those of secondary pulmonary artery hypertension; however, the perfusion lung scan in the former is normal; therefore a perfusion lung scan can be used to differentiate the two entities.
Diagnosis: Pulmonary emboli coexistent with venous thrombosis of lower extremity; unresolved perfusion defects.
Case 2.3
History: A 63-year-old alcoholic with hepatic cirrhosis and multifocal atrial tachycardia was referred for V/Q scintigraphy to rule out PE (Figs. 2.3 A, B).
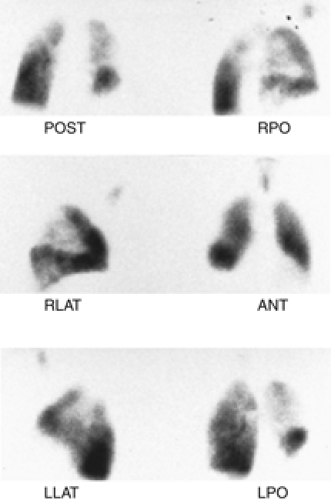
Findings: There is a large, well-defined, wedge-shaped perfusion defect involving the right upper lobe best seen in the lateral projection (Fig. 2.3 B), in the area in which there is some ventilation (Fig. 2.3 A). The radiographic abnormality (Fig. 2.3 C) is smaller than the perfusion abnormality.
Discussion: A perfusion defect substantially larger than the chest x-ray opacity with preservation of some ventilation is one of the criteria for a high-probability scan.
Given this patient’s history of alcohol use and esophageal varices, he is considered to be at high risk for anticoagulation. The PE diagnosis was confirmed by angiography (Fig. 2.3 D) and a Greenfield filter was inserted.
A perfusion defect in an area of pulmonary parenchymal opacity does not mean an intermediate probability for PE in every instance. The scan can be classified as high probability when the criteria are met. In this case, the presence of some
ventilation supports the diagnosis of PE. The segmental nature and wedge-shaped defect extending to the pleural surface is also a characteristic pattern for PE and should not be weighed lightly where the diagnosis of PE is concerned.
ventilation supports the diagnosis of PE. The segmental nature and wedge-shaped defect extending to the pleural surface is also a characteristic pattern for PE and should not be weighed lightly where the diagnosis of PE is concerned.
In patients with a high-probability scan and a relatively high clinical likelihood of PE, there should be no need for further diagnostic tests; however, in patients undergoing more aggressive therapy, such as inferior vena cava filtration, thrombolysis, or embolectomy, pulmonary angiography is indicated and justified.
Diagnosis: High probability of acute PE.
Case 2.4
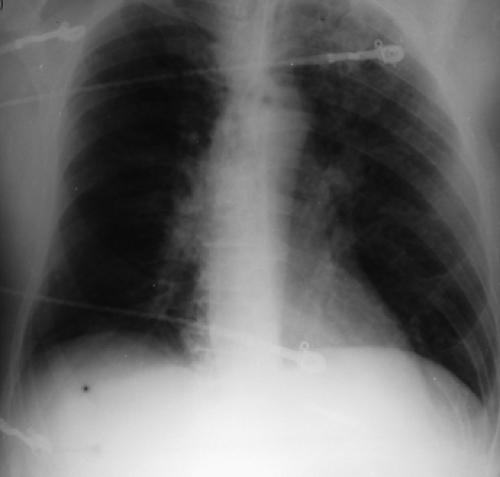
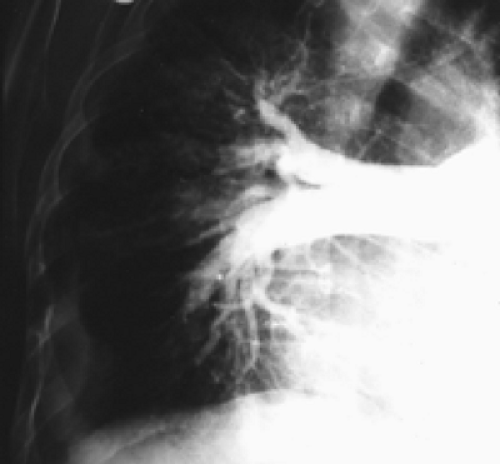
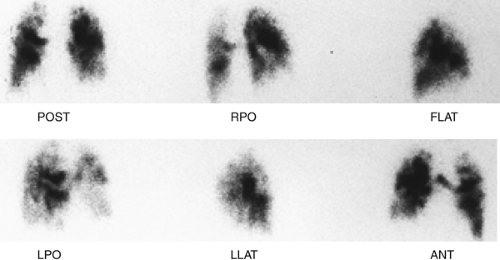
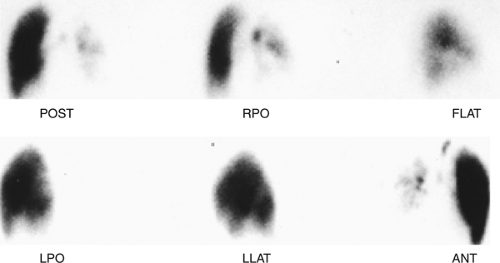
History: A 62-year-old man presented to the emergency room with dyspnea, chest pain and hypoxemia. Acute myocardial infarction was excluded and an emergency V/Q scan was performed (Figs. 2.4 A and 2.4 B). An admission chest x-ray was taken (Fig. 2.4 C).
Findings: The chest x-ray reveals enlargement of the right pulmonary artery but no parenchymal infiltrates. The lung scan shows diffuse heterogeneous distribution of radioaerosol and a large mismatched perfusion defect involving virtually the entire right lung.
Discussion: A scintigraphic finding of near absence of right lung perfusion in an adequately ventilated lung implies a high probability for PE. Other causes of a near absence of unilateral lung perfusion defect include the following: bronchogenic carcinoma causing compression of the pulmonary artery; congenital heart disease and after shunt procedure; fibrosing mediastinitis usually due to histoplasmosis; postradiation fibrosis; unilateral hyperlucent lung (Swyer-James syndrome); pulmonary arterial hypoplasia; giant bullae; mediastinal hematoma resulting from aortic dissection (valve replacement); Marfan’s syndrome; and several other uncommon conditions. Most of these entities can be excluded by history and radiographic findings.
In addition to the clinical presentation, the enlargement of the pulmonary artery (Fleischner’s sign) seen on this patient’s chest x-ray is a supportive finding that the V/Q mismatch represents a pulmonary embolus for which the patient received treatment. Enlargement of the pulmonary artery is due to pulmonary arterial hypertension and/or distention of vessels by bulky thrombosis. It is to be noted that prominence of the pulmonary artery may be seen in 20% of radiographs of patients with or without pulmonary emboli. As such, the sensitivity of Fleischner’s sign in predicting PE is low and many patients with PE do not show this sign.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree