, Joon Woo Lee1 and Jong Won Kwon2
(1)
Department of Radiology, Seoul National University Bundang Hospital, Seongnam, Kyonggi-do, Republic of Korea
(2)
Department of Radiology, Samsung Medical Center, Seoul, Republic of Korea
18.1 Neurofibromatosis
18.2 Marfan Syndrome
18.7 Renal Osteodystrophy
18.9.1 Neurofibromatosis
18.9.2 Marfan Syndrome
18.9.6 Spinal Arachnoid Cysts
18.9.7 Renal Osteodystrophy
18.9.8 Extramedullary Hematopoiesis
Abstract
This chapter will discuss miscellaneous spinal disorders which have characteristic imaging findings. These include neurofibromatosis, Marfan syndrome, idiopathic hypertrophic pachymeningitis, spontaneous intracranial hypotension, superficial siderosis caused by spinal tumor, spinal arachnoid cysts, renal osteodystrophy, and extramedullary hematopoiesis.
This chapter will discuss miscellaneous spinal disorders which have characteristic imaging findings. These include neurofibromatosis, Marfan syndrome, idiopathic hypertrophic pachymeningitis, spontaneous intracranial hypotension, superficial siderosis caused by spinal tumor, spinal arachnoid cysts, renal osteodystrophy, and extramedullary hematopoiesis.
18.1 Neurofibromatosis
Spinal manifestations in neurofibromatosis type 1 are skeletal anomaly such as severe scoliosis, multiple intradural and extradural neurofibromas, dural ectasia with lateral meningocele (common in thoracic spine), gliosis or rarely astrocytomas in the spinal cord (high signal on T2-weighted images), and multiple cutaneous neurofibromas (Khong et al. 2003; Egelhoff et al. 1992).
Spinal manifestations in neurofibromatosis type 2 are multiple spine tumors: extradural schwannomas, intradural extramedullary meningiomas or schwannomas, and intramedullary ependymomas. Skeletal anomaly and dural ectasia is uncommon in neurofibromatosis type 2 (Egelhoff et al. 1992).
Table 18.1
Spinal manifestations of neurofibromatosis
Neurofibromatosis type 1 | Neurofibromatosis type 2 | |
|---|---|---|
Extradural tumor | Neurofibromas | Schwannomas |
Intradural extramedullary tumor | Neurofibromas | Meningiomas, schwannomas |
Intramedullary lesion | Gliosis, rarely astrocytoma | Ependymomas |
Others | Scoliosis | |
Dural ectasia | ||
Lateral meningocele |
18.2 Marfan Syndrome
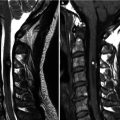
Marfan syndrome is an autosomal dominant hereditary disorder of connective tissue that affects primarily the eye, the skeleton, and the cardiovascular system. Scoliosis occurs in half of patients with Marfan syndrome. Dural ectasia, ballooning or widening of the dural sac, may develop in two-thirds of patients, with scalloping of the posterior aspects of one or more vertebral bodies, particularly in the lumbar region (Habermann et al. 2005). Dural ectasia can be diagnosed accurately with MR imaging and CT. Dural ectasia may be seen in other disorders such as neurofibromatosis type 1, Ehlers–Danlos disease, and ankylosing spondylitis. A rare but serious complication is atlantoaxial subluxation.
18.3 Idiopathic Hypertrophic Pachymeningitis
Idiopathic hypertrophic pachymeningitis is a chronic progressive inflammatory disorder and localized or diffuse thickening of the dura mater that leads to spinal cord compression (Ranasinghe et al. 2011). This rare disorder is usually found intracranially, and the spinal form is extremely rare. Idiopathic hypertrophic spinal pachymeningitis is a diagnosis of exclusion. Various disease entities causing diffuse dural thickening, such as meningitis, metastasis, and subdural hematoma, should be excluded. Idiopathic hypertrophic spinal pachymeningitis is manifested in radiculopathy, myelopathy, or a combination of both, secondary to nerve root and spinal cord compression. It usually involves the cervical and thoracic dura. The dorsal dura is more commonly thickened than the ventral aspect, with the thickness varying from 5 to 15 mm. Corticosteroids have been considered as the main conservative treatment; however, relapses are common. Patients with linear dural enhancement have better response to the corticosteroid treatment than with nodular dural enhancement. Surgical decompression can alleviate symptoms and neurologic sequelae. Currently, decompressive surgery for symptomatic compressive lesion combined with steroid therapy for the inflammatory process is the mainstay of treatment (Pai et al. 2007).
On MR imaging, idiopathic hypertrophic pachymeningitis has been described as a dural-based mass of T2 hypointensity extending over multiple levels with strong enhancement. The spinal cord may show diffuse atrophy associated with long-standing compressive myelopathy. Pathological confirmation is required for establishment of the diagnosis. In addition to thickening and fibrosis of the dura, histology shows inflammatory cell infiltration, which is composed of lymphocytes, plasma cells, and occasional foreign-body giant cells.
18.4 Spontaneous Intracranial Hypotension
Spontaneous intracranial hypotension (SIH) most commonly results from CSF leaks in the spine. Causes of CSF leaks include the following: lumbar puncture, surgery, trauma and connective tissue disorders such as Marfan syndrome. Targeted epidural blood patches can be effective in the treatment of SIH; thus it is important to precisely localize the site of CSF leakage. CT myelography is useful in detecting the site of CSF leakage. Contrast that has leaked outside the dura shows lower attenuation than contrast remaining in the subarachnoid space (“gray-rim appearance”). Contrast leakage may also be seen as a diverticulum around the nerve root or as extravasation into the paravertebral space. MR myelography with heavily T2-weighted images can also be used to detect CSF leakage without the need for contrast injection into the subarachnoid space.
18.5 Superficial Siderosis Caused by Spinal Tumor
Superficial siderosis of the central nervous system results from deposition and accumulation of iron salts along the surfaces of the brain, spinal cord, or cranial nerves and the hard iron salt core developing from chronic bleeding into the subarachnoid space or on the brain surface. The cerebellum is the organ most affected by superficial siderosis. Patients most frequently present with slowly progressive gait ataxia and sensorineural hearing impairment. A clinical history of subarachnoid hemorrhage is often absent. MRI evaluation for hemosiderin deposition is based on demonstration of magnetic susceptibility along the surfaces of the cerebellum, cord, cerebrum, cisternal portions of the cranial nerves, and intrathecal spinal nerve roots. Magnetic susceptibility is seen as signal intensity dropout on T2-weighted images and is accentuated on gradient echo sequences where the signal dropout is more conspicuous. The most common underlying cause of superficial siderosis is chronic bleeding into the subarachnoid space. The chronic bleeding may arise from various sources such as an arteriovenous or cavernous malformation, spinal tumor, chronic subdural hematoma, ventricular shunting, chronic subarachnoid hemorrhage, past brain surgery, or CNS trauma. Spinal tumors associated with superficial siderosis due to chronic bleeding include ependymomas (most common spinal tumor causing superficial siderosis), meningeal melanocytomas, paragangliomas, and pilocytic astrocytomas (Salem et al. 2002; Spengos et al. 2007; Matsumoto et al. 1998; Sharma et al. 1998; Bostantjopoulou et al. 2000). As such, in patients with superficial siderosis without a discernible intracranial cause, whole spine imaging is needed to find the source of chronic bleeding.
18.6 Spinal Arachnoid Cysts
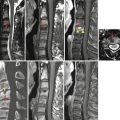
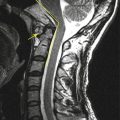
Spinal arachnoid cyst can be seen in the intradural or extradural location. Intradural arachnoid cyst is usually small, less than one or two spine segments, and located in the dorsal aspect of midthoracic level in most cases. Because the wall of intradural arachnoid cyst is very thin, current imaging modalities cannot delineate the wall in most cases. So, the imaging features suggestive of intradural arachnoid cyst include absence of cerebrospinal fluid (CSF) flow artifact in dorsal subarachnoid space inside the cyst with spinal cord compression. On CT myelography, there is a filling defect in the arachnoid cyst. Some arachnoid cyst can communicate with subarachnoid space, so in those cases, the diagnosis is difficult. The differential diagnoses of intradural arachnoid cyst are spinal cord herniation, subarachnoid web, and intradural adhesion. The most important finding to suggest intradural arachnoid cyst is a round area with absence of flow artifact inside the dura with cord compression.
Extradural arachnoid cyst is caused by arachnoid herniation through dural defect. Extradural arachnoid cyst is commonly located in the thoracolumbar junction. Because of CSF flow pulsation, the extradural arachnoid cyst can become large with adjacent bone erosions. Extradural arachnoid cyst can extend to the paraspinal area through neural foramen. CT myelography can confirm the extradural arachnoid cyst because in CT myelography, the injected contrast is seen in the extradural arachnoid cyst.
Table 18.2
Intradural and extradural arachnoid cyst
Intradural arachnoid cyst | Extradural arachnoid cyst |
|---|---|
Midthoracic level | Thoracolumbar level |
Dorsal subarachnoid space | Epidural and paraspinal |
Small | Large |
Spinal cord compression | Bone erosion, foraminal widening |
Absence of CSF flow artifact on MR | Contrast inside the cyst on CT myelography |
18.7 Renal Osteodystrophy
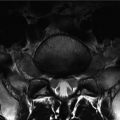
Renal osteodystrophy is a group of secondary musculoskeletal abnormalities that result from the metabolic disorder of calcium and phosphate caused by chronic renal failure. Osseous abnormalities of renal osteodystrophy include secondary hyperparathyroidism, rickets and osteomalacia, osteosclerosis, osteoporosis, fractures, and soft tissue calcification (Jevtic 2003). Secondary hyperparathyroidism may manifest radiographically as osteosclerosis, osteopenia, and subchondral bone erosion. Subchondral bone resorption in sacroiliac joints leads to widening of the joint space. Osteomalacia refers to the softening of the bones caused by insufficient or delayed mineralization of osteoid tissue and manifests as osteopenia, a decrease in bone density and blurred, coarse bone trabeculae. Osteosclerosis due to renal osteodystrophy tends to predominate in the axial skeleton, most commonly manifesting in the pelvis, ribs, and spine. The physiology of osteosclerosis associated with renal failure is complex. Osteoblasts increase the amount of osteoid in response to bone resorption and a subsequent loss of bone mass. This excess osteoid appears radiopaque, thus producing the rugger jersey spine appearance (band-like increased opacities along the superior and inferior aspect of the vertebral bodies). The rugger jersey spine sign is almost diagnostic of the osteosclerosis associated with secondary hyperparathyroidism of chronic renal failure (Wittenberg 2004).
18.8 Extramedullary Hematopoiesis
Extramedullary hematopoiesis refers to the formation of blood cells occurring outside of the bone marrow. Physiological extramedullary hematopoiesis occurs in the spleen and liver during fetal development. Hematopoiesis shifts to the bone marrow prior to birth. Hematological or marrow disorders associated with poor blood cell formation will cause conversion of yellow marrow to red marrow throughout the skeleton. If red marrow reconversion is insufficient, hematopoiesis will shift outside of the bone marrow, mostly in the liver, spleen, and kidney (Ginzel et al. 2012). Extramedullary hematopoiesis can rarely occur in paraspinal or epidural space. The extraosseous extension of medullary tissue derived from vertebral bodies may be detected with MR imaging. Epidural hematopoiesis may have mass effect on spinal cord. The masses show intermediate signal intensity on both T1- and T2-weighted MR images, similar to that of hematopoietic bone marrow. The signal intensity of the vertebral bodies is also diffusely decreased on both T1- and T2-weighted images secondary to chronic anemia. After gadolinium administration, enhancement is minimal or absent, differentiating it from other paraspinal lesions such as metastases or abscesses. Inactive lesions show hyperintensity on both T1- and T2-weighted images due to fatty infiltration or hypointensity on both T1- and T2-weighted images due to iron deposition (Haidar et al. 2010). Bony destruction or pathological fractures are usually absent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree