Characteristic
Median recurrence risk (%)
Range (%)
References
Clinical N0
2
0–9
<5 metastatic nodes
4
3–8
>5 metastatic nodes
19
7–21
Clinical N1
22
10–42
Clinical N1 with extranodal extension
24
15–32
19.3 Postoperative Surveillance
19.3.1 Risk of Recurrent Disease
The term recurrent disease is often used interchangeably with persistent disease, though the two entities are distinct [43]. A recurrence is defined as the appearance of tumor in a patient previously thought to have no evidence of disease [43]. Persistent disease is that which is identified prior to a patient being labeled as having no evidence of disease [43]. Historically, thyroid cancer has a high rate of recurrent disease, up to 30 %, at 40 years of follow-up [44]. This data, however, is derived from utilization of historically more insensitive means for detecting residual disease, including palpation of the neck and diagnostic whole-body scans [44]. Palpation examination may miss nearly 40 % of nodal disease subsequently detected with sonography [2, 3]. Furthermore, diagnostic whole-body scans fail to identify many metastatic lesions now recognizable with serum Tg measurement and US examination of the neck [45]. Therefore, the majority of “recurrences” identified by Mazzaferri et al. [44] more likely represented persistent disease that was unable to be distinguished with the imperfect modes of detection available at the time. Moreover, the recurrent disease probably took many years of growth to come to clinical detection. With improved preoperative sonographic detection of metastatic disease, modern patients are undergoing more extensive initial surgeries [46]. And, with very sensitive means of detection of residual thyroid tissue , once a patient is labeled as being free of disease, it seems unlikely that the rates of recurrence with long-term follow-up will remain as high as 30 %. More recent short-term estimates of recurrence rates are very low, though long-term follow-up with the current imaging modalities and ultrasensitive Tg measurements remains unavailable [6, 47–49].
The majority of recurrences in patients with papillary thyroid cancer involve locoregional lymph nodes. Sonography of the neck is a critical tool for detection of these metastases as diagnostic whole-body scanning and palpation are very insensitive [2, 3, 45]. For this reason, sonography of the neck is an indispensable component of the long-term follow-up in patients with papillary thyroid cancer . The optimal timing of the initial postoperative ultrasound examination and the subsequent need for repeat examinations is dependent on the initial risk of recurrence [1]. This stratification system is outlined in the sections below. The ultrasound examination is thus an important component of the determination that a patient has been rendered free of disease [1].
Tumor recurrence rates escalate with increasing tumor size; in one study those tumors less than 1 cm had a 10-year recurrence risk of 4.6 %, and the risk increased incrementally to 24.8 % in those larger than 8 cm [50]. Additional factors impacting recurrence include tumor histology, patient age, extent of nodal involvement, and possibly BRAF mutation status [1]. Variable rates of recurrence risk across studies may also stem from the vigor with which one searches for residual disease. The contemporary definition of biochemical cure should include both imaging and biochemical parameters, however. The current criteria for disease-free status from the ATA, now termed an “excellent response to therapy,” include no clinical or imaging evidence of tumor and a serum Tg level during TSH suppression of <0.2 ng/mL or after stimulation of <1 ng/mL in the absence of interfering antibodies [1]. The frequency for performance of neck sonography in patients with differentiated thyroid cancer should be adapted to that individual’s risk of structural recurrence. Therefore it is important for clinicians to understand the benefits and limitations of staging systems to predict recurrence.
19.3.2 Staging Systems
The tumor, node, and metastasis (TNM) system proposed by the American Joint Committee on Cancer (AJCC) is the most widely used staging system for thyroid cancer and is designed to predict risk of mortality [51]. Other staging systems, including European Organisation for Research and Treatment of Cancer (EORTC) [52], Age, Metastasis, Extent of disease, Size (AMES) [53], and Metastasis, Age at presentation, Completeness of surgical resection, Invasion (extrathyroidal), Size (MACIS) [54], were created to capture additional clinicopathologic factors that impact survival such as patient age or tumor invasiveness. Numerous studies have demonstrated that the TNM and MACIS systems offer the most reliable estimates of mortality [55, 56]. These systems have significant shortcomings, however. One major limitation is that no single staging system adequately captures all tumor and patient features that impact prognosis. Consequently, the ability of any of these staging systems to accurately predict death from thyroid cancer in the individual patient is limited [55, 56]. The clinician should be aware of these limitations when discussing the tumor stage with a patient, regardless of the system employed.
Fortunately, prediction of mortality is typically a secondary concern for most patients with differentiated thyroid cancer; the risk for recurrence is more probable and consequently of more immediate interest. Because the TNM system does not accurately differentiate the risk of recurrence among the vast majority of patients who are either stage 1 or stage 2, the ATA developed a three-tiered classification of tumors based upon surgical pathology features [1]. Differentiated thyroid cancers are categorized as low, intermediate, or high risk for structural recurrence based on various tumor features that are obtained on surgical pathology. Small, intrathyroidal tumors with minimal nodal involvement are at low risk for recurrence, between 1 and 8 % [1]. Intrathyroidal tumors less than 4 cm with vascular invasion, clinically evident nodal disease, greater than five nodal metastases , or BRAF mutation have an intermediate risk of recurrence, between 10 and 30 % [1]. Papillary cancers larger than 4 cm with BRAF mutations or gross extrathyroidal extension, tumors with nodal disease measuring >3 cm or extranodal extension, and follicular carcinomas with extensive vascular invasion are deemed high risk with a recurrence rate between 40 and 50 % [1]. This stratification system not only aids the clinician in determining the need for additional therapy but also helps to guide the initial timing and intensity of surveillance and the level of TSH suppression in the first 12–18 months postoperatively [1].
19.3.3 Initial Ultrasound Surveillance
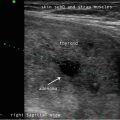
Active surveillance for recurrent disease initially combines measurement of serum thyroglobulin and ultrasound of the neck [1]. At a minimum, ultrasound of the neck should be performed at 6–12 months after surgery for papillary thyroid cancer and then periodically thereafter, depending on the patient’s risk for recurrence and thyroglobulin status [1]. The central and lateral cervical nodal compartments should be interrogated by an experienced sonographer utilizing a high-frequency probe (≥10 MHz) [1].
19.3.4 Long-Term Ultrasound Surveillance
It is important to recognize that the risk of recurrence may change over time depending on the clinical course of the disease and the response to therapy [1]. A dynamic stratification system has been proposed to assess the response to treatment and hence modify the initial structural recurrence risk estimation [57, 58]. This system may be used at each visit to reassess an individual’s likelihood of recurrent disease; the information is then incorporated into the surveillance strategy and guides the extent of TSH suppression and other treatment decisions, the most common of which is frequency of neck sonography [1, 57]. The system has been retrospectively validated in two studies [57, 58].
Response to therapy is divided into one of four groups: excellent, biochemical incomplete, structural incomplete, and indeterminate [1]. An excellent response to therapy is typified by a lack of clinical, biochemical, or structural evidence of disease. Initially low- and intermediate-risk patients who achieve this status have a very low risk of recurrence, between 1 and 4 %. In contrast, high-risk patients to reach this benchmark have a risk of recurrence of 14 % [57]. A biochemical incomplete response is marked by an abnormal thyroglobulin value (suppressed serum Tg between 1 and 10 ng/ml or stimulated Tg >10 ng/ml) or rising anti-Tg antibody level in the absence of visible disease [57]. During follow-up, approximately 30 % of these patients will be reclassified as having no evidence of disease, 20 % will achieve disease-free status after additional therapy, and 20 % will develop structural disease during follow-up [57]. Those with persistent visible disease are classified as structural incomplete response to therapy [57]. The majority of these patients, 50–85 %, will have persistent disease despite additional therapy. Finally, an indeterminate response to therapy is denoted by nonspecific structural findings that cannot be confidently viewed as malignant or benign, a low-level detectable suppressed Tg (greater than the low limit of normal for the assay but less than 1 ng/ml), a stimulated Tg <10 ng/ml, or declining anti-Tg antibody levels [57]. During follow-up, 15–20 % of these patients will have structural disease identified, and 80–85 % will have stable or resolving clinical findings [57].
The response to therapy stratification is used to guide the clinician in long-term follow-up and management decisions, including the intervals for US surveillance. In patients with an excellent response to therapy, a reduction in the intensity and frequency of follow-up is appropriate [1]. In fact, low-risk patients with no detectable disease can be followed primarily with clinical examination and serum Tg measurement while on levothyroxine replacement therapy [1]. Because initially high-risk patients that achieve an excellent response to therapy may have a higher risk of recurrence, a more intensive follow-up strategy may be appropriate, with yearly follow-up and periodic US examinations for the first 5 years after achieving disease-free status. Patients with a biochemical incomplete response to therapy may require additional imaging if the Tg or Tg-antibody titer is rising [1]. If a structural incomplete response is seen, continued imaging with US is appropriate for determination of rate of growth and potentially the development of new lesions [1]. Those patients with an indeterminate response to therapy and nonspecific findings on US may require serial sonographic imaging to identify changing features or growth [1].
19.4 Observation of Small Nodal Disease
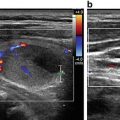
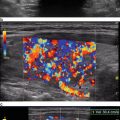
Sonography is very sensitive for identifying minimal residual thyroid tissue and nodal metastases as small as 2–3 mm in diameter. These tiny foci may be very difficult to identify intraoperatively, however. And the clinical impact of this minimal residual disease is questionable [1]. Nearly half of the “suspicious” US findings that are aspirated are ultimately deemed to be benign [59–61]. Additionally, several studies have demonstrated that the majority of suspicious lesions in the postoperative neck do not demonstrate significant growth [62–64]. Though the percentage of lesions that exhibited growth was variable in the studies, it remained low, between 9 and 20 %, even with long-term follow-up (3.5–14 years) [62–64]. The studies consistently found that the variable which best predicted the growth of a lesion was the largest lesional diameter; bigger masses were more likely to grow [62, 64]. Even among the minority of malignant lesions that demonstrated enlargement, the rate of growth was typically slow, with a 1.3 mm/year increase [64]. Fortunately, when the structurally progressing lesion was ultimately resected, there was no evidence of local invasion or distant metastases [63, 64]. Taken together, these data point to the conclusion that with careful sonographic follow-up including serial measurement of Tg, observation rather than surgical removal may be warranted in properly selected patients with minimal residual disease [1, 43]. Alternatively, those sonographically suspicious lesions measuring greater than 8–10 mm in smallest diameter may be considered for fine-needle aspiration with cytology and thyroglobulin measurement in the needle washout fluid and, ultimately, removal [1]. Suspicious lesions that are smaller than 8–10 mm also may be considered for intervention if there is growth or if the node threatens vital structures [1].
19.5 Limitations of Ultrasound Imaging for Cervical Lymph Nodes
Clinicians should be cognizant of the limitations of neck sonography. First, sound waves cannot penetrate air, bone, or cartilage. Consequently, nodes posterior to the trachea may be difficult to identify. Second, the depth of a structure may exceed the limits of high-resolution sonography. As a result, the superior mediastinal and deep level IV lymph nodes may be difficult to see, particularly in patients with a large body habitus. Third, artifacts from normal anatomic structures may also obscure visualization of nodal metastases. For example, isoechoic nodes that lie posterior to a large vessel may be difficult to delineate. The posterior acoustic enhancement associated with the sound waves traveling through the vessel may disguise an isoechoic metastatic node.
Computerized tomography may therefore improve the sensitivity of identifying disease in the retropharyngeal space and the superior mediastinum [65] and may be a useful adjunct to ultrasonography in preoperative planning [1]. Indeed, the combination of CT and US was found to have increased sensitivity to either modality alone for the identification of nodal disease in the central compartment [66].
Extrathyroidal extension may be seen in up to 15 % of patients with papillary thyroid cancer , with the severity ranging from microscopic invasion to gross extension of the tumor into the surrounding vital structures and soft tissues [67]. Though gross extrathyroidal extension may be identified on ultrasonography [68], invasion into the trachea or esophagus cannot be assessed sonographically. CT offers improved visualization of the integrity of these structures [66, 69]. Knowledge of the extent of tumor invasion could significantly impact the surgical approach [70].
Another limitation of sonography includes the identification of lesions and structures not related to the cancer but which may be confused with a recurrence. These include suture granulomata, neuromas, parathyroid adenomas, thymic tissue, and metastases from other primary tumors, to name a few [71, 72]. The distinction between benign and malignant nodes may also be confusing, particularly with plump reactive nodes in which the characteristic hilar stripe cannot be readily identified. These often may be seen in level II, as repeated microtrauma in the mouth chronically stimulates the node and causes resultant hypertrophy [71, 72]. The distinction between suspicious and malignant lymph nodes is discussed in greater detail in Chap. 20.
19.6 Summary
Ultrasound plays an important role in the diagnosis and surveillance of patients with thyroid cancer. Preoperative US of the neck is a critical component of the surgical planning; identification of metastatic nodes changes the operative approach. Sonography also helps identify recurrent disease in the neck in the postoperative patient; all patients should have a surveillance US 6–12 months after surgery. The importance of the ultrasound examination in the management of thyroid cancer patients is further established in that it is a central component of the definition of freedom from disease. The necessity of, and interval for, subsequent imaging is based on the response to therapy. Though sonography is pivotal to the evaluation and surveillance of thyroid cancer, there are important limitations to its use. The clinician should not only have expertise in the distinction of those metastatic foci of disease but also have a facile understanding of the mimics of thyroid cancer recurrence. Furthermore, when suspicion is high for the presence of persistence or recurrence in the absence of a sonographic suspect lesion, other imaging modalities may be necessary to examine the regions of the neck that are not adequately visualized sonographically.
References
1.
Haugen BR, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.CrossRefPubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree