The development in neuroimaging techniques has revolutionized the way neurology is practiced, including neurologic disorders in tropics. Some diseases occur exclusively, whereas some are more common in tropical regions. However, some are becoming increasingly prevalent in the developed world too, as a result of patterns of human migration and globalization. It is imperative to learn about the role of imaging in tropical neurology, which might assist early diagnosis and treatment and also add to the existing knowledge. Infections are more common in the tropics and require special attention in view of their potential treatability.
Common infections affecting the nervous system in the tropics
Cerebral Malaria
Clinical features, natural history, and prognosis
Cerebral malaria occurs as a result of Plasmodium falciparum malaria that causes unconsciousness from widespread brain disease affection. If left untreated, it is a fatal disease in 25% to 50% of patients within 72 hours. Neurologic sequelae are associated with protracted seizures, prolonged and deep coma, hypoglycemia, and severe anemia. Some of the neurologic deficits like ataxia are transient, whereas others like hemiparesis and cortical blindness may not improve incompletely. There are reports of African children with severe spastic tetraparesis and vegetative states, who usually die within a few months of discharge. Those who survive might have sequelae in the form of persistent neurocognitive defects and language and behavioral problems (especially among survivors in sub-Saharan Africa). The histopathologic signature is sequestration of cerebral capillaries and venules with parasitized red blood cells (RBCs) and nonparasitized RBCs. The specific treatment consists of chemotherapy with quinine or artemisinin (artesunate and artemether) and several adjunctive measures (ie, management of hypoxemia, hypoglycemia, hypovolemia, shock, anemia, metabolic acidosis, seizures, and neuroprotective therapy).
Role of imaging in cerebral malaria
Imaging has a lesser role to play in the diagnosis and management of cerebral malaria. However, magnetic resonance (MR) imaging studies offer a better understanding of the pathogenesis of cerebral malaria. Almost all MR imaging studies to date have involved a single case report or small series of patients. The first MR imaging study of a series of patients with malaria living in an endemic area was performed using a 0.2-T scanner, which showed that cerebral edema is not a consistent feature in living patients with cerebral malaria. The other reported MR imaging findings noted are white matter changes and pontine signal alterations. A computed tomography (CT) brain scan has poor sensitivity compared with MR imaging and is reported to reveal diffuse cerebral edema. In a CT pathologic study of 21 patients with cerebral malaria, the scan did not show features like petechial hemorrhages in any of the 7 patients on whom an autopsy was performed. Newer MR imaging techniques like susceptibility sequences show diffuse petechial hemorrhages throughout the gray-white matter junction, corpus callosum, and internal capsules. Techniques like diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping might unravel restricted or free diffusion, whereas diffusion tensor imaging analysis might show additional white matter changes in patients with cerebral malaria, which might provide better understanding.
Central Nervous System Amebiasis
Central nervous system (CNS) infections in humans caused by free-living amoebae of the genera Naegleria or Acanthamoeba were first described in 1965 by Fowler and Carter and in 1966 by Butt. Naegleria and Acanthamoeba organisms are responsible for causing primary Acanthamoeba meningitis (PAM) and granulomatous amoebic encephalitis (GAE), respectively, having distinctive epidemiology, pattern of presentation, clinical course, pathology, and imaging findings. GAE is a subacute to chronic infection caused by Acanthamoeba and also Leptomyxida organisms. Acanthamoeba species are found in all types of environments. Cases have also been associated with amoebic keratitis from contaminated contact lens solutions or with hematogenous spread from a primary source of infection, either a pulmonary focus or a skin ulcer. GAE is known to occur in patients who are debilitated or immunocompromised by AIDS, chemotherapy, or steroid therapy. The clinical course is characterized by a long duration of focal neurologic symptoms unlike the rapid progression and fulminant course of patients with PAM. GAE is diagnosed by identifying Acanthamoeba trophozoites or cysts in cerebrospinal fluid (CSF)/brain biopsy. Cultures of brain tissues or CSF can also reveal Acanthamoeba organisms. The identification of amoeba requires immunofluorescence, immunohistochemistry, or DNA probes. Direct fluorescent antibody studies confirm the species type. The mainstay of treatment of PAM is amphotericin B, rifampicin, and miconazole.
Role of imaging in CNS amebiasis
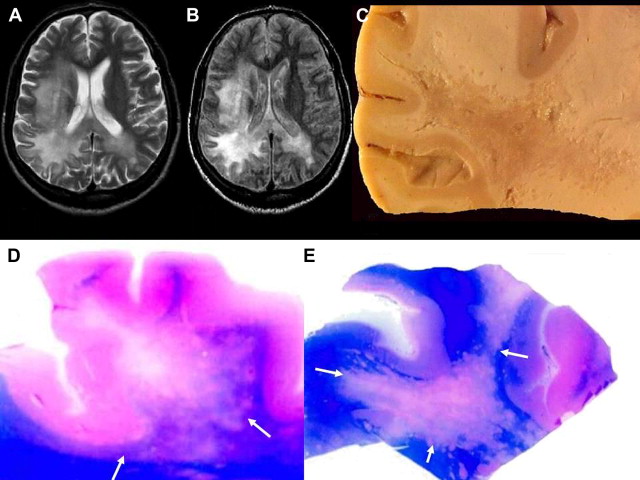
Imaging features of PAM are nonspecific and, to our knowledge, have rarely been described previously. Findings of CT and MR imaging may be normal early in the disease, with evidence of brain edema and basilar meningeal enhancement. Follow-up CT and MR imaging might reveal a pattern of brain edema and hydrocephalus, with rapid progression of the disease. There are reports of obliteration of the cisterns with enhancing basilar exudates. Rarely infarction of the right basal ganglia, possibly because of obliteration of the perforating vessels by the extensive exudates, has been described ( Fig. 1 ).
Neurocysticercosis
Clinical features, natural history, and prognosis
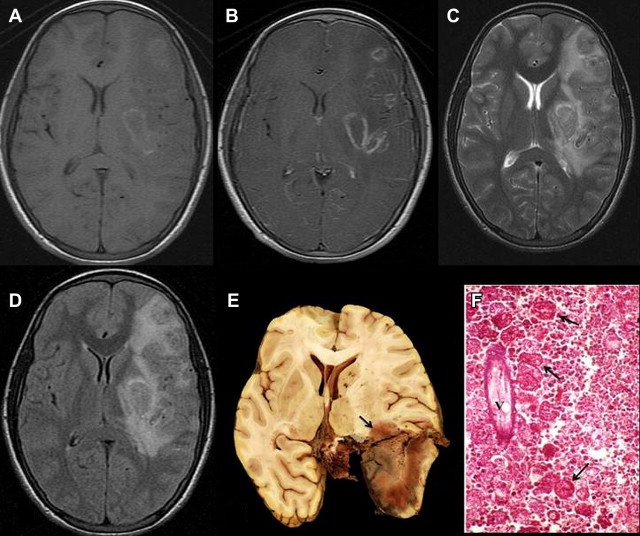
Neurocysticercosis (NCC) is an important cause of neurologic morbidity in developing countries, and is becoming increasingly prevalent in the developed world too, because of current patterns of human migration. It is the most common parasitic disease affecting the brain. Humans are the definitive host and pigs are the intermediate host for Taenia solium. If humans ingest T solium eggs, they develop cysticercosis. Larvae penetrate the intestinal wall and are carried to many tissues, where cysticerci develop. Neurologic manifestations are most common and include seizures caused by inflammation surrounding cysticerci in the brain, features of increased intracranial pressure (ICP), meningitis, and uncommonly other focal neurologic deficit. Unlike patients from Latin America and Africa, Indian patients with NCC commonly have single or a few intraparenchymal cysts with minimal recurrence of seizures. In tropical countries like India, NCC is one of the commonest causes of acute symptomatic seizures (localization related). The clinical manifestations are caused by deposition of larvae of the parasite T solium in cerebral parenchyma, meninges, spinal cord, muscles, eyes, and skin. Around 60% to 70.8% of NCC in India is in the form of solitary cerebral cysticercal lesion (SCCL). The diagnosis of cerebral NCC can be arrived at by good-quality CT and MR imaging. Serologic tests provide additional support in establishing the diagnosis. Histopathologic examination of lesion may rarely be required to prove the diagnosis. The anticysticercal drugs albendazole and praziquantel are variably effective for all forms of NCC. However, there is a controversy regarding its usage in both SCCL and malignant multiple NCC. Effective preventive health measures are safe drinking water, good hygiene, avoiding uncooked and contaminated food, and proper disposal of excreta. The diagnosis of NCC has been greatly facilitated by the advent of reliable imaging using CT and MR imaging. Radiological descriptions assist in clinical classifications of NCC based on the topography and stage of the lesions, and determining the rational therapeutic approach for the different forms of the disease ( Fig. 2 ). Imaging features of NCC are described in detail in the section on parasitic diseases.
Parasitic Eosinophilic Meningitis
Clinical features, natural history, and prognosis
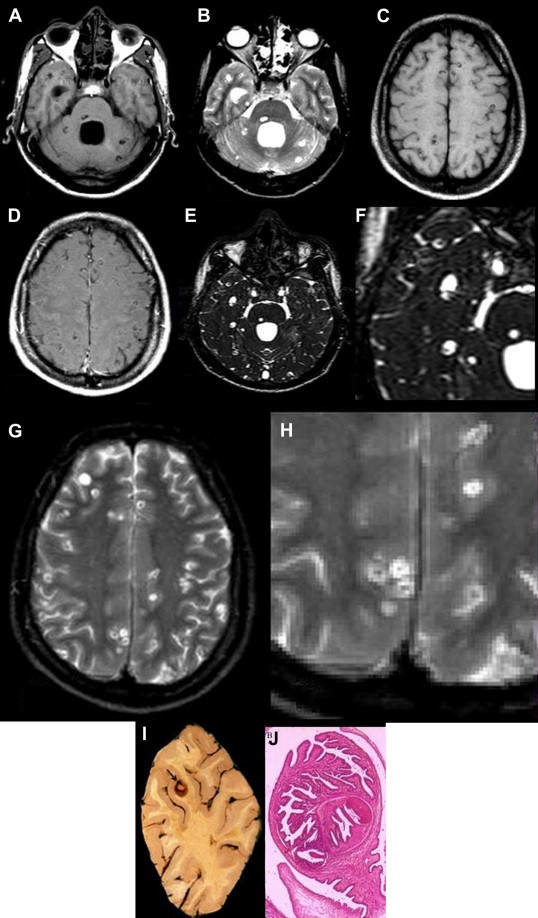
Angiostrongylus cantonensis, also known as the rat lungworm, is the most common cause of eosinophilic meningitis worldwide. This parasitic infection is endemic in the Southeast Asian and Pacific regions. The intermediate hosts are raw fish and snails. The typical clinical presentation is acute meningitis with an eosinophilic pleocytosis frequently accompanied by encephalopathy. The pathologic findings in the CNS include the following: (1) meningitis with a predominance of eosinophils and plasma cells, (2) tortuous tracks of various sizes in the brain and spinal cord surrounded by an inflammatory reaction and degenerating neurons, (3) granulomatous response to the dead parasites, and (4) nonspecific vascular reactions including thrombosis, rupture of vessels, arteritis, and aneurysm formation.
Role of imaging in parasitic eosinophilic meningitis
The brain CT scan can be normal or can reveal cerebral edema, ventricular dilatation, or enhancing ring or disc lesions, resembling tuberculoma. The features of the brain MR imaging scan were previously limited to a few case reports. These reports revealed vascular thrombosis, tortuous tracts in the vicinity of small vascular lesions, adjacent tissue reaction, and meningeal enhancement. MR imaging findings in CNS infection with A cantonensis are nonspecific, ranging from normal to leptomeningeal enhancement, ventriculomegaly, punctate area of abnormal enhancement, and hyperintense signal lesions on T2-weighted images. There seems to be a special predilection for involvement of the globus pallidus and cerebral peduncle in some patients. This finding is correlated with presence of worms in the CSF, severity of headache, CSF pleocytosis, and CSF and peripheral eosinophilia.
CNS Tuberculosis
Clinical features, natural history, and prognosis
The various forms of CNS tuberculosis include tubercular meningitis (TBM); tuberculoma parenchymal or extraparenchymal, intraventricular, basal cisternal; tubercular cerebritis or abscess; and spinal cord tuberculosis especially spinal arachnoiditis. The clinical features of TBM differ from other bacterial meningitis in that it has a subacute course (days to weeks), high mortality, less CSF pleocytosis, and higher risk of sequelae despite treatment. The prodrome includes headache, vomiting, fever, irritability, and insomnia for 2 to 3 weeks. Then there are neck stiffness, seizures (especially in children), cranial nerve syndromes (late), stupor, and coma. Fever (which initially may be low grade), irritability, stiff neck, Kernig and Brudzinski signs, papilledema, cranial nerve abnormalities (especially third nerve palsy), and other focal signs. Later apathy, confusion, lethargy, and stupor might be observed. CSF shows increased pressure, slightly cloudy, moderate pleocytosis (100–500 cells/mm 3 , mostly lymphocytes), increased protein, and decreased glucose. Growth on CSF cultures is rare. Confirmation of CSF for tuberculosis could be by (1) stained smear identifying acid-fast bacilli; (2) polymerase chain reaction (PCR) for mycobacterial DNA; or (3) mycobacterial culture. The treatment includes initiation with 4 oral drugs (rifampin, isoniazid, pyrazinamide, ethambutol) with steroids for the initial 2 months. Subsequently rifampin and isoniazid might be required for a period of another 10 months or so. Multidrug resistance tuberculosis is increasing and requires 5 or 6 drugs and a longer-term treatment regime. Brain tuberculosis may occur in a variety of forms on MR imaging, including meningitis, tuberculomas, cerebritis, and frank abscesses. Tuberculous meningitis is most commonly seen as a communicating hydrocephalus with diffuse enhancement disproportionately affecting the basal cisterns ( Fig. 3 A–D). The role of imaging in CNS tuberculosis is described separately elsewhere in this issue.
Acute Bacterial Meningitis
Clinical features, natural history, and prognosis
Acute bacterial meningitis is characterized by acute onset of intense headache, fever, nausea, vomiting, photophobia, and meningism (ie, neck stiffness or Kernig sign). Neurologic signs include lethargy, delirium, coma, and convulsions. Focal deficits are uncommon in pyogenic meningitis. Acute complications include increased ICP, seizures, sepsis, paralysis, SIADH (syndrome of inappropriate antidiuretic hormone hypersecretion). The common organisms differ by age, for example, adults: Streptococcus pneumoniae , then Neisseria meningitidis , then cryptogenic (organism not identified); neonates: group B streptococci, Escherichia coli ; children: Hemophilus influenzae . Acute meningeal syndrome mandates emergency neuroimaging, lumbar puncture (if not contraindicated), and emergent empiric intravenous antibiotic therapy. Initial antibiotic therapy is third-generation cephalosporin and vancomycin; ampicillin is added, if Listeria monocytogenes is considered. Definitive therapy is guided by organism and identification of source of infection. The mortality of acute bacterial meningitis is about 21% (depending on the organism) and occurs mostly within the first 48 hours.
Role of imaging in acute bacterial meningitis
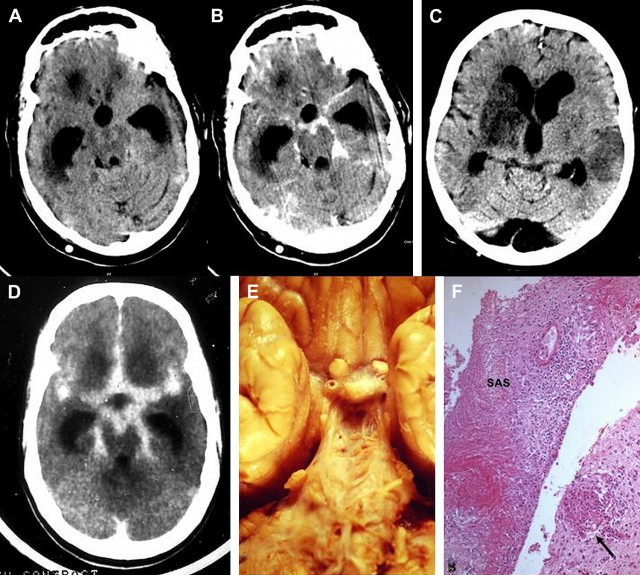
Neuroimaging studies have important adjunctive roles in identifying the complications of bacterial meningitis, such as hydrocephalus, subdural effusions, or subdural empyema, and in detecting parameningeal abscesses or CSF leaks. Indications for neuroimaging in acute bacterial meningitis include depressed level of consciousness, prolonged, partial, or late seizures, focal neurologic deficits, enlarging head circumference, persistent or recurrent fever during the later stages of treatment, and recurrent meningitis, sometimes before performing even lumbar punctures. Neuroimaging studies can also improve clinicians’ ability to prognosticate. Imaging should be considered strongly in neonates with bacterial meningitis, especially when gram-negative organisms are identified. Although noncontrast conventional MR imaging in uncomplicated infectious meningitis is usually normal, prominence of subarachnoid space or increased signal of the cisterns may occasionally be seen on proton density images. Fluid-attenuated inversion recovery (FLAIR) is the most sensitive MR imaging sequence for the evaluation of intracranial infections and has higher sensitivity than T2-weighted imaging for detecting meningitis. Abnormalities on FLAIR images include hyperintensity of the subarachnoid space and hyperintense vessels. On postcontrast MR imaging scans, homogeneous leptomeningeal enhancement of the falx, tentorium, convexities, and basal cisterns may be noted. Intravascular enhancement may also be seen. Abnormal meningeal or intravascular enhancement, or FLAIR subarachnoid hyperintensity on MR imaging, may occur in a variety of conditions other than infectious meningitis. Conventional MR imaging and CT are more useful tools for the diagnosis of the complications of meningitis, such as ventriculitis, hydrocephalus, infarction, cerebritis/abscess, myelitis, and subdural empyema. The use of DWI and magnetization transfer may increase the sensitivity of MR imaging in diagnosing infectious meningitis and its ischemic complications. Newer techniques like three-dimensional constructive interference in steady state and MR cisternography could be used to delineate CSF leakage in patients with recurrent meningitis ( Fig. 4 A–D).
Brain abscess
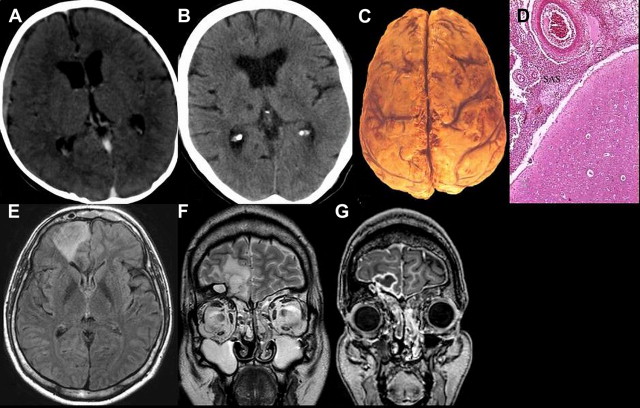
Neuroimaging studies are essential for making a diagnosis of brain abscess. They reveal a focal process that rapidly assumes the character of a mass lesion with a contrast-enhancing margin. If MR imaging is not available, CT is the preferred procedure. Perilesional mass effect and even midline shift are often noted. Hyperintensity on DWI with a reduced ADC is characteristic of brain abscess. In the absence of restricted diffusion on DWI, in vivo proton MR spectroscopy can distinguish brain abscesses from cystic tumors. In patients with cyanotic congenital heart disease, brain abscess must be differentiated from intracranial vascular accidents and hypoxic attacks (see Fig. 4 E–G).
Neurosyphilis
Clinical features, natural history, and prognosis
Syphilis, a potentially treatable condition, involves the nervous system at multiple neuraxes and thereby has protean manifestations. It was common in the first half of the 20th century, but after effective use of penicillin, its incidence has decreased considerably. However, in the last decade, there has been a 50-fold increase in its incidence in Eastern Europe. Further, in the human immunodeficiency virus (HIV) era, besides an increased incidence, it has acquired a fulminant course. According to a World Health Organization estimate, the total number of new cases of syphilis in the world in 1999 was 12 million, of which 90% were from developing countries. The classically described phases of the illness are asymptomatic, meningovascular syphilis, tabes dorsalis, general paralysis of insane, and gummata.
The spectrum of neurologic manifestations includes meningitis, stroke, myelopathy, cranial nerve involvement, symptoms of demyelination, seizures, and headache, which can be confused with many neurologic diagnoses. A high index of suspicion and the clinician’s awareness are thus important in diagnosis of neurosyphilis. CSF Venereal Disease Research Laboratory test is the most widely used, whereas the FTA-abs (fluorescent treponemal antibody absorption) test in the CSF is a highly sensitive marker for the presence of neurosyphilis.
Role of imaging in neurosyphilis
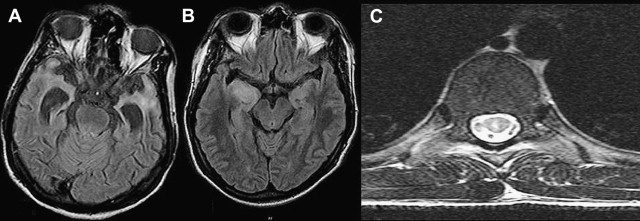
Neuroradiological findings in neurosyphilis may vary with the stage of the disease and meningitis, cranial neuritis, cerebrovascular disease, cerebral atrophy, or parenchymal gumma. Orbital involvement may also occur, particularly of the roof and supraorbital rim. The cerebrovascular disease is characterized by endarteritis, with neuroimaging studies typically revealing multiple areas of ischemia and infarction. Syphilitic gummas appear as discrete masses, ranging from a few millimeters to several centimeters in diameter and typically involving the cerebral cortex. Single-photon emission computed tomography (SPECT) studies reveal thalamic hyperperfusion in the acute stage, which is replaced by hypoperfusion in subacute or chronic stage. Rao and colleagues reviewed 35 MR images in patients with neurosyphilis. MR imaging of the brain was abnormal in 27 and revealed cerebral atrophy and signal changes in 18 each ( Table 1 ). MRA revealed basilar artery occlusion in one. In the myelopathy group, MR imaging of the spine showed signal changes, involving more than 3 vertebral segments, in thoracic cord in 7 patients. One of the 3 patients with normal MR spine imaging had medial temporal involvement, and 1 each with cord abnormalities had multiple lacunes and temporal lobe changes. In a proper clinical context, presence of signal changes in medial temporal and posterior column of spinal cord, especially with unexplained CSF pleocytosis, might suggest the diagnosis ( Fig. 5 ).
| MRI Abnormalities | N (35) |
|---|---|
| Abnormal | 27 |
| Neuropsychiatric manifestations | 17 |
| Cerebral atrophy | 15 |
| Diffuse | 13 |
| Fronto-temporal | 02 |
| Signal changes | 11 |
| Medial temporal | 03 |
| Fronto-temporal | 03 |
| Multifocal | 03 |
| Parietal | 01 |
| Basal-ganglion | 01 |
| Cerebellum | 01 |
| Meningo-vascular manifestations | 08 |
| Diffuse Cerebral atrophy | 03 |
| Signal changes | 06 |
| Medial temporal | 01 |
| Multifocal | 05 |
| MRA – basilar occlusion | 01 |
| Myelopathy manifestations | 10 |
| Hyperintense signal changes | 09 |
| >3 vertebral segments | 07 |
| <3 vertebral segments | 02 |
Nervous System Involvement in HIV
Clinical features, natural history, and prognosis
Involvement of the nervous system at autopsy is noted in about 80% to 90% of HIV-seropositive patients. There are reports of neurologic disorders as the presenting initial manifestation in 5% to 10% of patients with AIDS. Although seizures could be caused by HIV infection per se, they are more commonly observed in underlying opportunistic infections (OIs), systemic illness, drug or alcohol abuse, and even antiretroviral usage.
Role of imaging in HIV
The imaging changes in HIV-seropositive patients in tropical countries, although similar to those described elsewhere, are complicated by the presence of OIs more often than elsewhere. However, often imaging shows evidence of both HIV-related and OI- related changes. Progressive multifocal leucoencephalopathy (PML) does cause white matter signal alterations suggestive of demyelination ( Fig. 6 ) and often causes diagnostic difficulties, especially when associated with OIs.