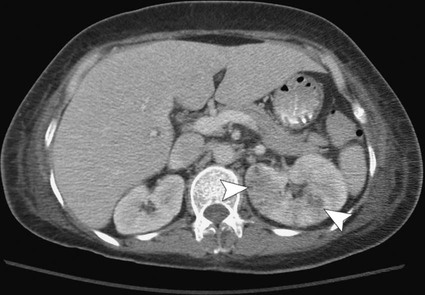
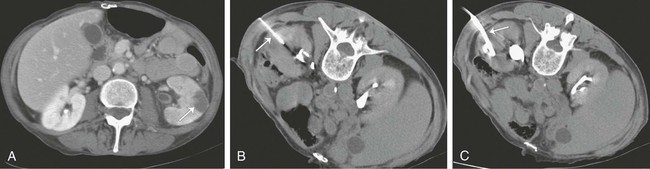
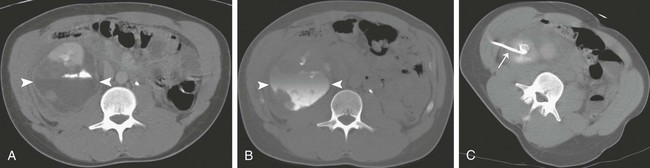
Percutaneous image-guided drainage is a useful technique for managing many fluid collections involving the upper genitourinary tract. For infected collections in the peritoneal cavity, percutaneous abscess drainage (PAD) has become the primary drainage procedure since its introduction in the early 1980s. This procedure’s safety and efficacy has been demonstrated in numerous large clinical trials.1–5 The logical extension of the technique to collections in the kidney and perirenal space has gained acceptance and in many cases obviated the need for urologic surgery. Benefits of percutaneous drainage of fluid collections in most cases include avoiding general anesthesia, laparotomy, and prolonged postoperative hospitalization, with resultant reduction in morbidity, mortality, and hospital costs. In this chapter, we address techniques for percutaneously draining renal and perirenal abscesses as well as other fluid collections such as hematomas and urinomas. Percutaneous nephrostomy techniques and ureteral stenting are addressed in other chapters. Percutaneous fluid drainage is a therapeutic option that lies between medical and surgical management. In general, the prerequisites for percutaneous renal and perirenal fluid collection drainage are identical to the indications for fluid collection drainage elsewhere in the abdomen—presence of a fluid collection and one or more of the following: suspicion the collection is infected, need for fluid characterization, or suspicion the collection is producing symptoms that warrant drainage.6 A few caveats should be added in the setting of suspected urinomas. The majority of small urinomas will resolve spontaneously, but if a small urinoma fails to resolve in several days or if a large urinoma is discovered, percutaneous drainage should be considered. Regardless of the collection size, if there is any suspicion a urinoma is infected, drainage should be pursued. The goal of drainage can be complete cure of a collection, or drainage may be employed as a temporizing measure before definitive surgical treatment. For example, in a critically ill patient with a suspected abscess in a nonfunctioning kidney, PAD can be performed to stabilize the patient before performing a definitive urologic procedure like nephrectomy. Similarly, treatment of urinomas caused by obstruction mandate not only drainage of the urinoma but also correction of the underlying leak and/or obstruction and reestablishment of urine flow to the bladder or to the outside for successful treatment of the urinoma. Contraindications to percutaneous fluid collection drainage are relative and should be weighed carefully against the suitable medical and surgical alternatives. According to the American College of Radiology (ACR) guidelines, the relative contraindications include coagulopathy that cannot be adequately corrected, hemodynamic instability that precludes completion of the procedure, inability of the patient to be positioned or cooperate adequately for the procedure, known adverse reaction to contrast medium when its administration is deemed necessary for success of the procedure, lack of a safe access route for drainage of a collection, and severely compromised cardiopulmonary status in patients undergoing procedures where risk or further cardiopulmonary compromise are inherent to the procedure.6 In each case, the risks of the procedure must be carefully assessed. The decision regarding management of renal and perirenal fluid collections is best made in concert with the surgical and medical teams caring for the patient. Lastly, every attempt is made to identify pregnant patients before their exposure to ionizing radiation. Pregnant patients undergoing procedures requiring use of ionizing radiation are counseled regarding the risk to the fetus of radiation exposure, as well as the clinical benefit of the procedure. Every attempt is made to avoid exposing the fetus to radiation in accordance with ALARA (as low as reasonably achievable) principles. Efforts to reduce radiation exposure for these patients include ultrasound guidance whenever possible. If computed tomography (CT) is required for a procedure, imaging is limited to only those areas of the body required to perform the procedure. Pus, urine, blood, and even pancreatic fluid can accumulate in the renal parenchyma and surrounding tissues. An understanding of distribution of the fluid in the renal and perirenal spaces must be founded on an understanding of the fascial planes that compose this area. The fascia that surrounds the kidneys envelops the perirenal spaces that contain the adrenal glands, proximal ureters, and fat that surrounds the kidneys. This perirenal fascia forms a long, tapered cone that reflects the embryologic ascent of the kidneys from the pelvis into their adult position in the retroperitoneum. The eponymous names of the anterior and posterior layers of the renal fascia are the Gerota fascia and Zuckerkandl fascia, respectively, but the name Gerota fascia is frequently used in the medical literature to describe both the anterior and posterior layers of renal fascia.7 The anterior and posterior layers fuse laterally to join the lateral conal fascia. Although there is no consensus on the strength or completeness of the inferior fascia, published reports suggest that inferior patency exists in some patients, allowing bidirectional flow of fluid between the perirenal and pelvic compartments.8–10 Similarly, although there is some controversy about the medial boundaries of the perirenal spaces, there is published evidence that patency between the right and left perirenal spaces across the midline exists at and below the level of the lower poles of the kidneys.9 The lower abdominal aorta and inferior vena cava are within this midline communication between the two perirenal spaces. Posterior to the perirenal space is the posterior pararenal space, which contains only a variable amount of properitoneal fat. This space is bounded posteriorly by the transversalis fascia and anteriorly by the perirenal fascia. Because this space contains only fat, it is rarely the primary site of a pathologic process. The perirenal fascia fuses at its posterior and lateral aspects to the surface of the pro-peritoneal fat. The fused surfaces of the posterior perirenal fascia and anterior surface of the posterior pararenal space create a potential space, the posterior interfascial or retrorenal plane, which may be filled from fluid arising in either of the compartments it bounds.11 Anterior to the perirenal space lies the anterior pararenal space that contains the ascending and descending colon, pancreas, and portions of the duodenum. As with the fusion of the posterior perirenal fascia and anterior surface of the posterior pararenal space, the fusion of the anterior perirenal fascia creates an additional potential space, the anterior interfascial plane or retromesenteric fusion plane, which can also be filled with fluid arising from the compartment it bounds.11 On the left, lateral in relation to the anterior renal fascia, the dorsal mesocolon fuses with the surface of the posterior pararenal fat to form the lateral conal fascia. Similarly, on the right, lateral in relation to the anterior pararenal fascia, the mesentery of the ascending colon fuses with the posterior renal fat to form the lateral conal fascia. Potential spaces are created with the formation of these interfascial planes as well. The retromesenteric anterior interfascial space, retrorenal posterior interfascial space, and lateral conal plane communicate at the fascial trifurcation.11 Within the perirenal space, the perinephric fat is divided by numerous thin fibrous lamellae into multiple smaller compartments that may or may not communicate. These fibrous structures are continuous with and interconnect the renal capsule and anterior and posterior renal fascia planes. These connections allow for bidirectional spread of blood, fluid, or edema from the perirenal fascial planes into the perinephric space.11 The terms bacterial nephritis12 and lobar nephronia13 were initially introduced to identify a subset of patients with acute pyelonephritis who had severe regional or generalized parenchymal abnormalities without abscess, a protracted clinical course, and eventual atrophy of the affected parenchyma. The majority of these patients were diabetic or immunocompromised. The goal of identifying this group of patients was to distinguish them from patients with uncomplicated acute pyelonephritis who responded rapidly to antibiotics, as well as from patients with renal and/or extrarenal abscesses. The term lobar nephronia was used to describe a lobar distribution of pathology. For the most part, infections of the renal parenchyma are the result of ascending spread of pathogens from the lower tract or hematogenous seeding of the renal parenchyma.14 In experimental models of acute ascending nonobstructive renal infection, vesicoureteral and intrarenal reflux have been demonstrated to carry bacteria to the pyelocaliceal system and papillary ducts and tubules, where an inflammatory response is initiated.15 Inflammation then radiates centrifugally from the papilla to the cortex along medullary rays in a lobar or sublobar distribution. The configuration of the duct openings at the papilla in part determines which papilla will allow reflux of urine and which will not.16 It was this proposed lobar distribution that gave rise to the term lobar nephronia.13 Bacterial nephritis was an additional term used for those patients who did not have lobar distribution of infection but who were likely to have a severe disease process with a protracted clinical course. Both of these terms have shortcomings. First, the literature now clearly demonstrates that otherwise healthy children and adults can have severe infections of the renal parenchyma that prolong treatment and result in parenchymal loss.17,18 Second, extensive experience with CT has now demonstrated that renal infections frequently do not correspond to a renal lobe. Although most renal infections are thought to be the result of an ascending infection, the extent to which reflux of urine is responsible for inoculation is unclear; acute pyelonephritis occurs in both adults and children, without evidence of reflux. One model used to explain this phenomenon is the ability of certain strains of virulent Escherichia coli to ascend the ureter against the flow of urine by the expression of surface adhesins. The bacteria then colonize the upper tract and penetrate parenchyma via collecting ducts and tubules.14 Furthermore, although less common, hematogenous spread of infection also accounts for many cases of renal infection. In animal models, bacteria reaching the renal parenchyma by this route begin in the cortex and involve the medulla within 24 to 48 hours. These lesions are typically multiple and rounded and have a nonlobar distribution throughout the periphery of the kidney. After 48 hours, however, distinguishing the two patterns is often not possible because spreading inflammation obscures the initial underlying pattern.19 To escape this nosologic quagmire in a way that reflects the underlying pathophysiologic process, takes into account the continuous nature of the spectrum of focal or diffuse renal infections, and allows the radiologist to convey an accurate, easily understood report of the process involving the kidney that will help guide therapeutic decisions, the Society of Uroradiology proposes a simplified nomenclature based on the term acute pyelonephritis.19 For CT imaging of patients clinically diagnosed with acute pyelonephritis, the society recommends that all regions of hypoattenuation visualized in the kidney be considered evidence of acute pyelonephritis (Fig. 147-1). The report should then further characterize the process as (1) focal (uni- or multi-) or diffuse, (2) unilateral or bilateral, (3) with or without focal or diffuse enlargement of the kidney, and (4) complicated (renal or extrarenal abscess, obstruction, gas formation) or not.19 If treated too late or inadequately, severe bacterial renal infections may progress to form an abscess. Initially, microabscesses will form that can then coalesce to form macroabscesses. Historically, pathologists and surgeons have used the term renal carbuncle to describe multiple coalescent hematogenously seeded renal abscesses. Over time, the term came to be used to describe any renal abscess. Although there is a distinctive macroscopic appearance to this entity, no corresponding imaging features have been described, so the term should be avoided by radiologists. A mature abscess will have a well-defined wall and on CT imaging will appear as an area of nonenhancement within renal parenchyma, with a peripheral rim of enhancement (Fig. 147-2). Before forming a mature abscess, however, there is an initial liquefaction of renal parenchyma in regions of inflammation. On CT, this can appear as an area of low attenuation measuring near water density that does not enhance. Importantly, this lesion may not yet be a drainable collection. When CT findings are indeterminate, needle aspiration can be performed. If less than 1 mL of fluid is aspirated, it is likely this lesion is not drainable and will heal with long-term antibiotic therapy.19 Abscesses in the perirenal space most commonly result from rupture of a renal abscess into the perinephric fat. Additional causes of perirenal abscesses include extension of abscesses from adjacent compartments into the perirenal space and superinfection of collections in the perirenal space (e.g., urinomas, hematomas). Continuous leak of urine into the perinephric fat will result in a collection of urine. These collections are most commonly referred to as urinomas, but other names have been used in the medical literature, including uriniferous perirenal pseudocyst.20 Urinomas are most commonly the result of forniceal rupture in the setting of obstructive uropathy. Other causes include both iatrogenic and noniatrogenic trauma (Fig. 147-3). Hematomas in the perirenal space have numerous causes. Abdominal trauma with resultant renal contusion, laceration, or fracture can cause a perinephric hematoma. Additionally, rupture of an aortic aneurysm can lead to a hematoma confined to the perirenal space.21 Spontaneous perirenal hematomas raise concern for an underlying neoplasm such as renal cell carcinoma or angiomyelolipoma.22 The underlying lesion may be small and can be obscured by the hematoma in the acute setting. Therefore, management of a completely spontaneous perirenal hematoma or a perirenal hematoma arising in the setting of minimal trauma always includes follow-up imaging until the hematoma has resorbed or another cause for the hemorrhage has been identified.
Renal and Perirenal Fluid Collection Drainage
Indications
Contraindications
Technique
Anatomy and Approaches
Renal and Perirenal Fluid Collection Drainage