Renal Failure
▪ RENAL FAILURE
There is no clearly defined set of biochemical or clinical criteria that characterize renal failure. Most authors use this term to describe a patient whose renal function is insufficient to maintain homeostasis. The term renal insufficiency characterizes a patient whose renal function is abnormal but capable of sustaining essential bodily functions. Uremia, the clinical syndrome that results from renal dysfunction, may be present in untreated patients with both renal insufficiency and renal failure. Uremia may result in symptoms related to a number of different organ systems including the gastrointestinal tract (nausea, vomiting), the cardiovascular system (hypertension, cardiac arrhythmias, pericarditis), the nervous system (personality changes, seizures, somnolence), and the hematopoietic system (anemia, bleeding diathesis). The term endstage renal disease is often used to describe a patient with chronic renal failure whose renal deterioration is irreversible and requires dialysis or renal transplantation to sustain life. There are a number of parameters that can be assessed to quantitate particular aspects of renal function. The most frequent with which radiologists deal is the glomerular filtration rate (GFR). This is usually expressed in mL per minute (volume of glomerular filtrate created per minute); the normal rate for an average-size adult is about 12 mL per minute. The rate varies directly with body size and diminishes normally with age. The most commonly used measure of glomerular filtration is a single determination of serum creatinine (creatinine is freely filtered, but neither secreted nor absorbed by renal tubules), but this assessment is not ideal: glomerular filtration may diminish as much as 50% below normal before serum creatinine rises, and glomerular filtration may change more rapidly than serum creatinine. Normal ranges for serum creatinine differ depending on body size, race, and sex. Determination of actual creatinine clearance rates is difficult, since it requires timed complete urine collections along with urine and serum creatinine levels; certain iodinated contrast agents and radiolabelled compounds can be used for the same purpose; an estimated glomerular filtration rate (eGFR) is sometimes approximated by formulae, such as the Cockroft-Gault method.
The RIFLE criteria divide degrees of renal failure into five clinical categories of increasing severity: risk (Cr 1.5X normal, GFR loss >25%, or urine output <0.5 mL/kg/hour × 6 hours), injury (Cr 2X normal, GFR loss >50%, or urine output <0.5 mL/kg/hour × 12 hours), failure (Cr 3X normal, GFR loss >75%, or urine output <0.3 mL/kg/hour × 24 hours or anuria × 12 hours), loss (persistent acute renal failure [ARF] >4 weeks), and end-stage kidney disease (complete renal functional loss >3 months).
Acute Renal Failure
ARF is the sudden rapid deterioration in renal function. Classically, the causes of ARF are divided into three broad categories: (1) prerenal, (2) renal, and (3) postrenal.
Prerenal causes are generally associated with volume depletion or renal hypoperfusion and are the most common causes of ARF. Such conditions include shock from sepsis, dehydration, burns, or hemorrhage; congestive heart failure; cirrhosis with ascites; diuretic use; and diabetic ketoacidosis. Acute renal arterial insufficiency or renal vein occlusion may also be responsible.
Renal causes for ARF may result from damage to any portion of the kidney (i.e., the tubules, the glomerulus, the interstitium, or the small vessels). Acute tubular necrosis (ATN) is among the most common of these causes. Interstitial causes for ARF include acute urate nephropathy, multiple myeloma, and acute interstitial nephritis. Glomerular damage may cause ARF as a result of acute glomerulonephritis, drug toxicity, Goodpasture syndrome, systemic lupus erythematosus, and other causes.
Postrenal ARF refers to the onset of renal failure secondary to acute ureteral or bladder outlet obstruction. Although postrenal causes of ARF account for only about 15% of the cases, this entity is the cause for ARF most frequently sought radiologically, both because acute obstruction represents an easily
reversed cause of acute renal dysfunction and because imaging is better able to diagnose obstruction than causes of renal or prerenal failure.
reversed cause of acute renal dysfunction and because imaging is better able to diagnose obstruction than causes of renal or prerenal failure.
Chronic Renal Failure
The gradual progressive loss of renal function characterizes chronic renal failure. The renal dysfunction is attributable to the loss of functioning renal parenchyma and is usually irreversible. The causes of chronic renal failure are protean, but they may be related to vascular disease (e.g., generalized arteriosclerosis and arterial infarction), intrinsic renal disease (e.g., chronic glomerulonephritis, autosomal dominant polycystic kidney disease, and interstitial nephritis), and systemic disease (e.g., diabetes mellitus and hypertension), or may be the result of long-standing obstruction (e.g., prostate hypertrophy, neurogenic bladder disease, and posterior urethral valves). The process of chronic renal failure often requires dialysis or renal transplantation.
Chronic renal failure, with or without dialysis, is often accompanied by renal osteodystrophy. The imaging findings include osteopenia, bone cysts, subperiosteal resorption, and multifocal regions of increased uptake on bone scans. There may also be calcification in the soft tissues and in the medium-size arteries more severe than that usually seen in atherosclerosis; the arterial calcification is correlated with an increased risk of the generalized cardiovascular disease, which chronic renal failure confers.
Quantitation of renal size is particularly important in evaluating patients with renal failure. In the absence of renal disease, overall renal volume increases throughout childhood, plateaus in early adult life, diminishes slightly in middle age, and shrinks more rapidly in the elderly. In morphologic examinations, renal volume may be approximated simply by measuring pole-to-pole length. Clearly, direct measurement of renal parenchymal volume by three-dimensional ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) more accurately assesses functional renal mass, and becomes even more accurate if the contents of the renal sinus and the volume of any dilated portions of the collecting system are excluded. Loss of parenchymal mass from any disease is irreversible, and since parenchymal volume is closely related to GFR, quantitation of parenchymal volume is important for prognosis. In some circumstances, renal mass is inversely related to function and prognosis, including the parenchymal hypertrophy that accompanies early diabetic nephropathy, and the progressive increase in renal volume seen in patients with autosomal dominant polycystic disease.
▪ IMAGING STUDIES IN RENAL FAILURE
Plain Radiography
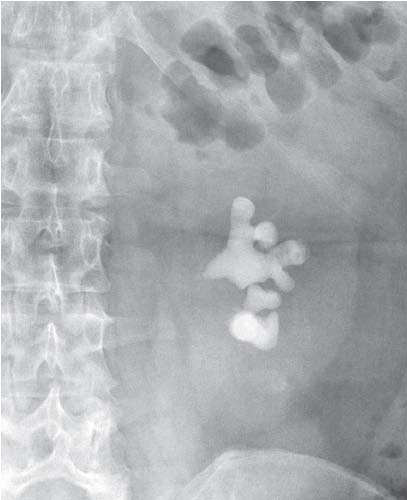
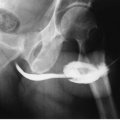
Radiographs can be used to detect obstructing stones (Fig. 11.1), parenchymal calcifications, and renal vascular calcifications, and may permit assessment of renal size. They may reveal abnormal gas collections in the patient with urosepsis, and allow assessment of the bony pelvis for renal osteodystrophy or metastatic disease.
The Nephrogram
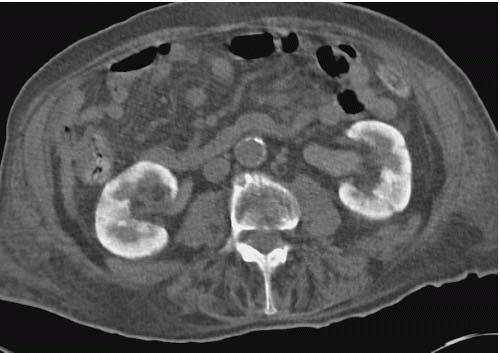
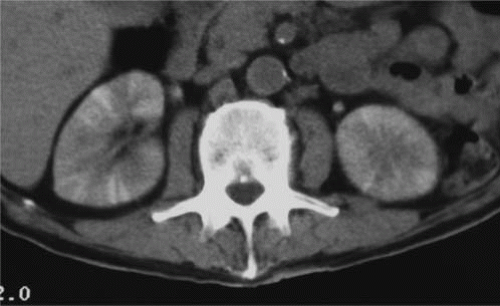
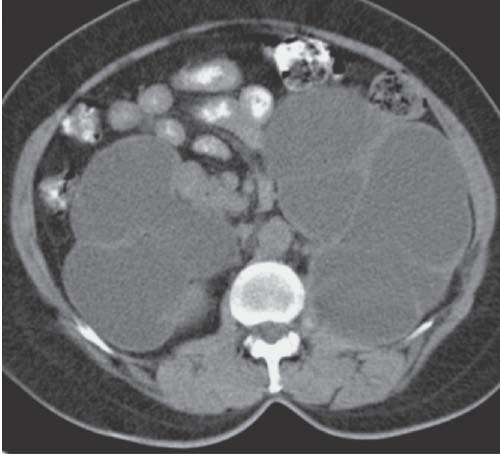
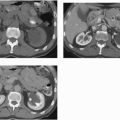
Renal parenchymal opacification (the nephrogram) is almost always reduced during contrast-enhanced CT, although much of the investigation of the renal parenchymal enhancement patterns seen in renal failure was originally performed with excretory urography. In both acute and chronic renal failure, the initial opacification of the renal cortex is slowed and diminished as compared with normal, and the washout, or deopacification, occurs at a slower rate than normal. In patients with chronic renal failure, the densest opacification of the cortical or medullary tissue is never as high as that seen in normal patients given the same contrast dose; this is probably due to a diminished GFR and diminished concentrating capacity of the remaining tubules. Patients with ARF share the slowed rate of opacification (Fig. 11.2) and deopacification of renal parenchyma seen with chronic renal failure, but the densest opacification reached is much more variable. In some patients, the parenchyma ultimately becomes very densely opacified and, in a few minutes or hours, becomes much denser than normal kidney tissue at the same intervals after contrast administration. This nephrogram may involve the cortex only (Fig. 11.3) or both the cortex and the medulla (Fig. 11.4), and is occasionally striated (Fig. 11.5). The mechanism of this dense prolonged nephrogram is not known with certainty; diminished flow rate of intratubular fluid resulting in abnormally high degrees of absorption of water leading to elevated intratubular contrast concentrations, leak of contrast-containing tubular fluid into the interstitium, and (in cases of acute obstruction) dilation of the lumina of contrast-containing tubules have all been postulated.
 FIGURE 11.2. ARF. Bolus-enhanced CT at the portal vein phase reveals almost no early renal parenchymal enhancement; normally there would be dense cortical enhancement. |
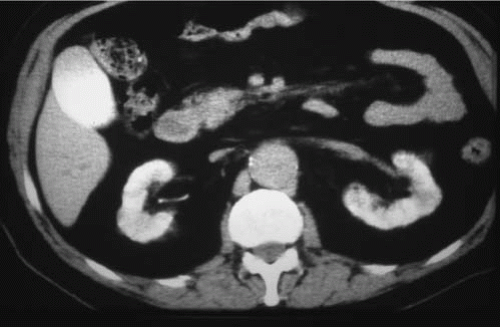 FIGURE 11.4. ARF. The renal parenchyma remains opacified, and there is vicariously excreted contrast in the gallbladder. |
The prolonged dense nephrogram of ARF may be encountered in prerenal, renal, or postrenal failure, and is often accompanied by some degree of oliguria. If it is bilaterally symmetrical, it is usually due to shock or ATN. A unilateral prolonged dense nephrogram is usually caused by acute ureteral obstruction—indeed, this nephrogram pattern is often called the obstructive nephrogram—but occasionally acute unilateral renal vascular insufficiency, due to conditions such as renal arterial embolization or dissection, or renal vein thrombosis, in which renal blood flow is diminished but not absent, may be causative. A dense prolonged nephrogram is never encountered in patients with chronic renal failure unless there is coexisting ARF.
A bilaterally symmetrical dense prolonged nephrogram in a radiograph or CT for which contrast has not been administered has been called a sentinel sign of ARF due to contrast administered for a prior recent examination, such as cardiac angiography. The delayed nephrogram of ARF is not always dense; it may be quite faint and never reach the density of the parenchymal opacification in normal kidneys.
Ultrasound
Ultrasound is the best initial imaging study for the patient with renal failure. In contrast to urography and, to a lesser extent, CT, ultrasound does not depend on renal function for the demonstration of renal anatomy. Therefore, in a patient with renal failure, sonography can easily distinguish a patient with normal-size kidneys, who usually has ARF, from one with small kidneys, and this generally indicates chronic renal failure (Fig. 11.6). Resistive indices are usually elevated in acute and chronic renal failure, but are usually normal in patients with prerenal failure. Sonography can also readily identify patients with autosomal dominant polycystic kidney disease and can accurately depict renal calculi as a cause of, or in association with, renal failure.
Ultrasound is effective in screening patients for urinary tract obstruction as the cause of renal failure. Obstructive renal failure is usually chronic in nature and is associated with hydronephrosis; it is therefore readily detectable by ultrasound (Fig. 11.7). In patients without known risk factors for urinary obstruction, the incidence of obstructive renal failure is relatively low. The accuracy of ultrasound in screening for chronic renal obstruction is discussed in Chapter 14.
Ultrasound may also provide limited information regarding the nature of the underlying renal disease. Most chronic renal parenchymal diseases result in increased cortical echogenicity (Fig. 11.8). Although such a finding has a high specificity, it has a relatively low sensitivity for detecting renal disease on screening sonography. Increased diffuse renal echogenicity, like diminished parenchymal volume, carries a poor prognosis for recovery of renal function. A small number of renal diseases including lymphoma, acute pyelonephritis, and renal vein thrombosis may cause decreased cortical echoes. Gouty nephropathy, medullary nephrocalcinosis, renal tubular acidosis, and medullary sponge kidney may result in increased medullary echogenicity.
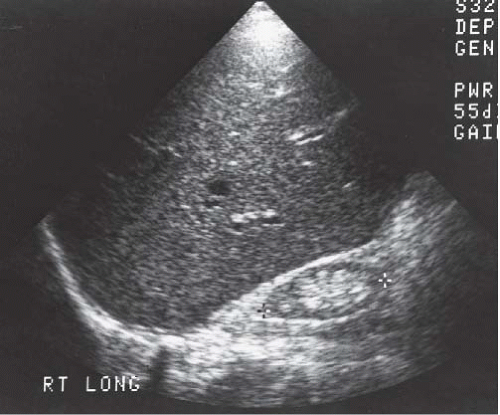 FIGURE 11.6. Chronic renal failure. Longitudinal sonogram of the right upper quadrant demonstrates a small echogenic kidney. |
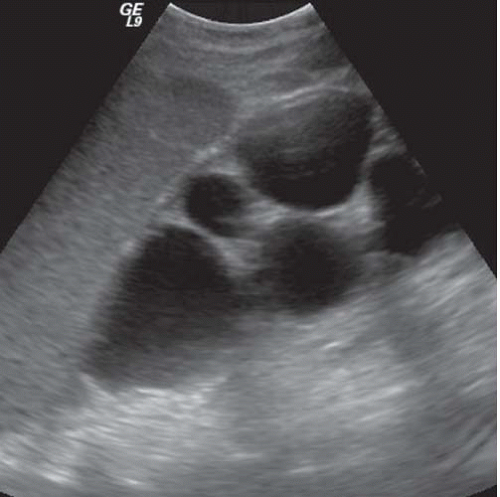 FIGURE 11.7. Hydronephrosis and obstructive atrophy. Longitudinal sonogram of the right kidney shows dilated calyces and thinned renal parenchyma. |
Computed Tomography
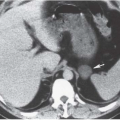
CT is often used in patients with renal failure when ultrasonography is inconclusive. Even without intravenous contrast administration, CT can detect hydronephrosis and can be useful in delineating the point and nature of an obstruction (Fig. 11.9). CT provides an accurate assessment of renal size and the degree of any cortical atrophy (Fig. 11.10). The degree of cortical atrophy is a good indicator of the amount of irreversible renal functional loss. If contrast is administered despite renal failure, the nephrogram will
be faint (Fig. 11.11). Cortical nephrocalcinosis indicates diseases such as Alport Syndrome, chronic glomerulonephritis, and oxalosis (Fig. 11.12), which produce renal failure. In some forms of renal cystic disease, CT is the imaging study of choice to detect a complication of the disease process (e.g., hemorrhage complicating adult polycystic disease or the development of a solid renal tumor in patients with acquired cystic disease). Finally, CT is highly sensitive for the detection of renal calculi.
be faint (Fig. 11.11). Cortical nephrocalcinosis indicates diseases such as Alport Syndrome, chronic glomerulonephritis, and oxalosis (Fig. 11.12), which produce renal failure. In some forms of renal cystic disease, CT is the imaging study of choice to detect a complication of the disease process (e.g., hemorrhage complicating adult polycystic disease or the development of a solid renal tumor in patients with acquired cystic disease). Finally, CT is highly sensitive for the detection of renal calculi.
Radionuclide Studies
Because the excretion of radiopharmaceuticals depends on renal function, they cannot be used to evaluate all patients with renal failure. This is particularly the case with technetium-99m diethylene-triaminepentaacetic acid (99mTc-DTPA), as it is excreted primarily by glomerular filtration. Technetium-99m-mercaptoacetyltriglycine (99mTc-MAG3), however, is excreted by tubular secretion and thus may demonstrate the kidneys even when renal dysfunction is relatively advanced. 99mTc-MAG3 uptake can be used to estimate function and is useful in predicting renal function that will remain after a unilateral nephrectomy. 99mTc-DMSA scans yield similar information. If obstruction is present, a percutaneous nephrostomy tube or ureteral stent should be placed and the kidney allowed sufficient time to recover maximal function before performing a radionuclide scan to estimate residual renal function.
 FIGURE 11.11. Chronic renal failure. Contrast-enhanced CT shows only faint enhancement of renal parenchyma. |
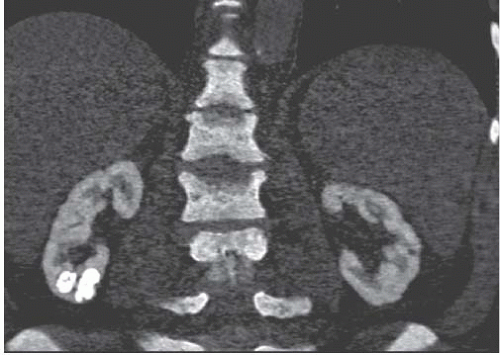 FIGURE 11.12. Oxalosis. Coronal reconstruction of noncontrast CT reveals diffuse renal cortical nephrocalcinosis and right lower pole oxalate stones. (Courtesy of Michael Morris, M.D.) |
Scintigraphy cannot always identify specific causes of renal failure because many abnormalities of perfusion, parenchymal uptake, and excretion are shared by many diseases; scan findings are often valuable. Moderate and severe parenchymal loss can be identified; bilaterally symmetrical parenchymal loss can distinguish small vessel, glomerular, and tubulointerstitial diseases from unilateral disease caused by large vessel diseases, ureteral obstruction, or scarring from stones or reflux. Unilateral persistent intense isotope uptake identifies disease caused by acute ureteral or large vessel obstruction. When bilaterally symmetrical, it is due to shock or acute renal or prerenal failure. Dilated pyeloureteral systems may be visible and indicate ureteral obstruction or severe reflux. Radionuclide determination of the GFR may also be of value (see Chapter 3).
Magnetic Resonance Imaging
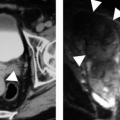
MRI provides useful information in some cases of chronic renal failure. The distinction between the cortex and the less-intense medullary pyramids usually seen in T1-weighted images of normal patients often disappears in patients with chronic renal failure, although the loss of the corticomedullary distinction may occur in normal patients who are well hydrated. Gadolinium enhancement of the parenchyma is reduced in chronic renal failure (Fig. 11.13). The superb anatomic detail afforded by MRI permits assessment of parenchymal volume (Fig. 11.14). Diseases that involve chronic hemolysis, such as hemoglobinopathies, prosthetic heart valves, and paroxysmal nocturnal hemoglobinuria (Fig. 11.15), may cause iron to be deposited in the renal cortex and produce low cortical signal intensity, especially with gradient-echo imaging. Hemochromatosis may produce similar signal loss in the medulla as well as in the cortex.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree