Fig. 1
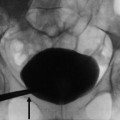
Young woman with acute focal pyelonephritis studied with ultrasound (US). (a) Ultrasound of the left kidney in a patient with fever and leukocytosis shows mild enlargement of the renal parenchyma with loss of corticomedullary differentiation in the middle part of the kidney (asterisk). (b) Contrast-enhanced ultrasound (CEUS) does not show any difference in the enhancement of the whole left kidney
Dilatation of the collecting cavities in the absence of demonstrable obstructive cause can be appreciated by US and correlate with the inhibition of normal ureteral peristalsis, due to the action of bacterial endotoxins.
In cases of fungal infection, US may demonstrate the presence of fungal debris with the formation of fungus balls in the pelvicalyceal system with consequent urinary obstruction. In addition, collection of air, due to gas-forming infections, may be seen in the bladder or collecting system (Vourganti et al. 2006).
Improvements in ultrasound techniques with power Doppler ultrasonography, demonstrating areas of parenchymal hypoperfusion, have resulted in better accuracy of US. Although experimental studies in animal models show a poor diagnostic accuracy in comparison with CT and scintigraphy, a positive power Doppler US can obviate the need for further examinations (Majd et al. 2001).
Contrast-enhanced ultrasonography (CEUS) has been recently reported as a useful tool to better demonstrate areas of poor perfusion related to pyelonephritis as a result of compression and vasoconstriction of arterial and arteriolar vessels, caused by inflammatory infiltration (Mitterberger et al. 2007). Comparative studies realized on animal models showed a significant increase of accuracy of the CEUS with respect to the power Doppler in the diagnosis of acute pyelonephritis, with values of sensibility comparable to those of MRI or CT imaging (Farhat et al. 2002).
However, this technique has not yet found a general use in the evaluation of the infected kidney and, in our experience, does not considerably improve the accuracy of US (Fig. 1b).
As stated by Granata et al. (2010), CEUS can be useful in acute pyelonephritis in kidney transplant patients.
According to the authors’ results, “CEUS may become the first-line diagnostic tool in this area because of its low cost and low toxicity, combined with a good diagnostic performance in terms of sensibility, specificity, accuracy and positive predictive value” (Granata et al. 2010).
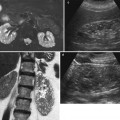
In our opinion, the main advantage of CEUS in acute pyelonephritis lies in excluding the growth of an abscess which requires a drainage in patients who does not respond to medical therapy (Fig. 2).
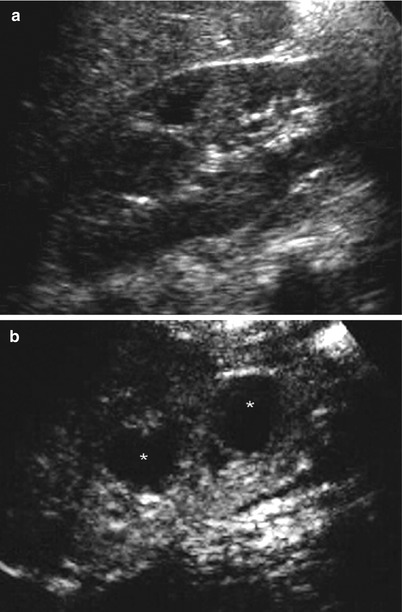

Fig. 2
Diabetic patient with right kidney abscesses. (a) Conventional ultrasound of the right kidney does not show any focal or diffuse lesion inside the renal parenchyma. (b) CEUS shows two round hypoechoic foci in the renal parenchyma (asterisks) suggestive of renal abscesses
CEUS is, conversely, a fundamental diagnostic tool to image pediatric patients suspected of having VUR, using first-generation or, preferably, second-generation contrast agents with urosonography (Ascenti et al. 2004; Zimbaro et al. 2007).
1.2.3 CT
CT is considered the most appropriate imaging technique for the diagnosis and follow-up of patients with acute bacterial pyelonephritis. Its main roles are to delineate the extent of the disease and to identify significant complications or urinary obstruction.
Unenhanced CT is extremely useful for the detection of renal enlargement, hemorrhage, inflammatory masses, obstruction, calculi, parenchymal calcifications, and urinary tract gas, and, therefore, it should be always performed first in the imaging protocol (Kawashima et al. 1997; Kawashima et al. 2000). Involved areas occasionally present with lower attenuation due to edema or necrosis; in rare hemorrhagic bacterial nephritis, unenhanced CT demonstrates wedge-shaped or rounded areas of increased attenuation determined by parenchymal bleeding (Rigsby et al. 1986; Craig et al. 2008) (Fig. 3).
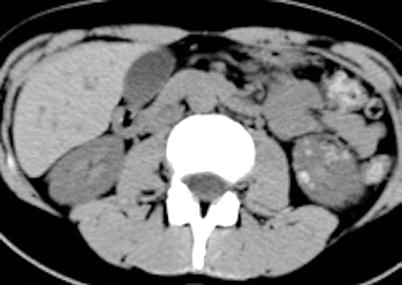

Fig. 3
Hemorrhagic acute bacterial pyelonephritis. Unenhanced computerized tomography (CT) examination shows multiple round or oval high-density foci within the left kidney (Reprinted, with permission from Craig et al. (2008))
However, the above-described findings are frequently absent; therefore, contrast-enhanced scans often have to be performed following the unenhanced study.
Helical and multidetector CT allows studying the different phases of excretion after intravenous contrast medium administration. A rational CT protocol for investigating patients with suspected acute pyelonephritis requires performing precontrast imaging followed by postcontrast acquisition at approximately 50–100 s after injection and continuing with delayed imaging especially if urinary tract obstruction is suspected (Stunel et al. 2007). These parameters are designed to take advantage of the nephrographic phase considered to be superior in depicting the full extent of lesions and to better define the characteristic abnormalities of acute pyelonephritis (Wyatt et al. 1995). Further, delayed CT scans at 3 h or more can help differentiate acute pyelonephritis from hypoattenuating areas caused by other pathologies like infarcts and tumors.
After the injection of contrast material, acute pyelonephritis usually appears as one or more wedge-shaped zones of lesser enhancement extending from the papilla in the medulla to the kidney capsule with a lobar distribution and poor corticomedullary differentiation. Enhancement of the involved region may be either homogeneous or irregular, but attenuation is always minor than normal parenchyma (Figs. 4a, b and 5).
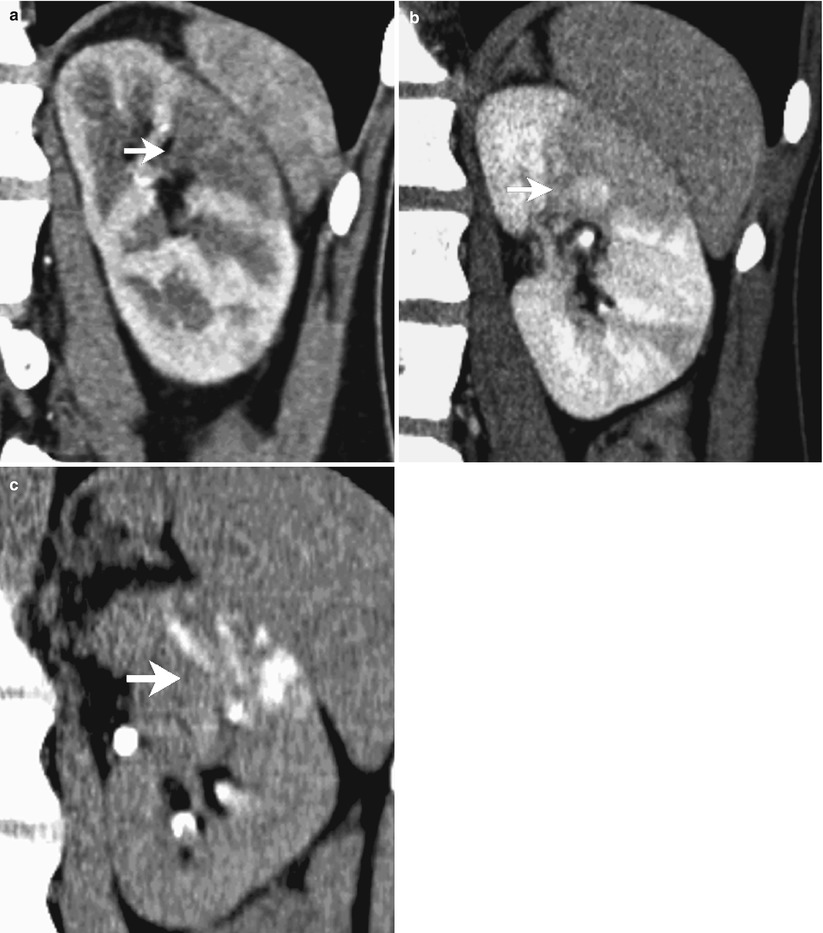
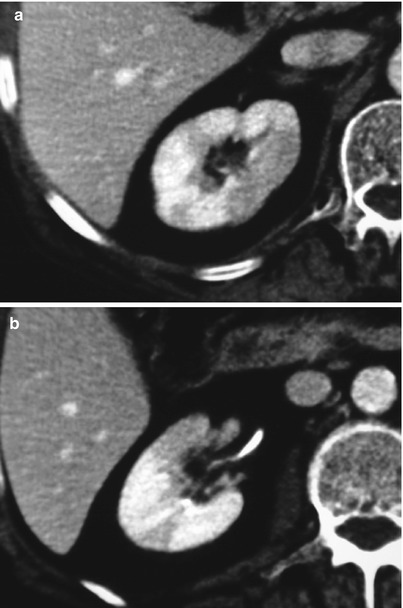

Fig. 4
Postcontrast coronal multiplanar reconstruction CT images in a patient with acute focal bacterial pyelonephritis of the left kidney. A wedge-shaped area of low attenuation radiating from the medulla to the cortical surface with loss of corticomedullary differentiation (arrow) is well demonstrated both in the arterial (a) and in the nephrographic phase (b) of the study. Delayed CT acquisition during the excretory phase (c) shows partial, persistent enhancement of the low-attenuation area (arrow)

Fig. 5
Male patient with multifocal acute bacterial pyelonephritis of the right kidney. Contrast-enhanced CT scans obtained at different levels show multifocal regions of diminished enhancement both in the upper (a) and in the middle part (b) of the right kidney
Vasospasm, tubular obstruction, and/or interstitial edema originates in areas of poor or nonfunctioning parenchyma which may determine this pattern of differential enhancement (Kawashima et al. 1997; Gold et al. 1983; Hoddick et al. 1983). The tendency of these three disorders to decrease the contrast agent flow through the tubule helps explain the pattern of delayed and persistent enhancement seen in delayed scans obtained 3–6 h after contrast medium administration (Dalla Palma et al. 1995) (Fig. 4c).
In acute pyelonephritis, the alternating bands of increased and decreased attenuation, which correspond to the differential enhancement of infected and not infected parenchyma, are sharply defined at the edge. During the healing process, the differential enhancement becomes more and more faint until it will either completely normalize or evolve to a scar, as demonstrated by parenchymal volume reduction (Fig. 6).
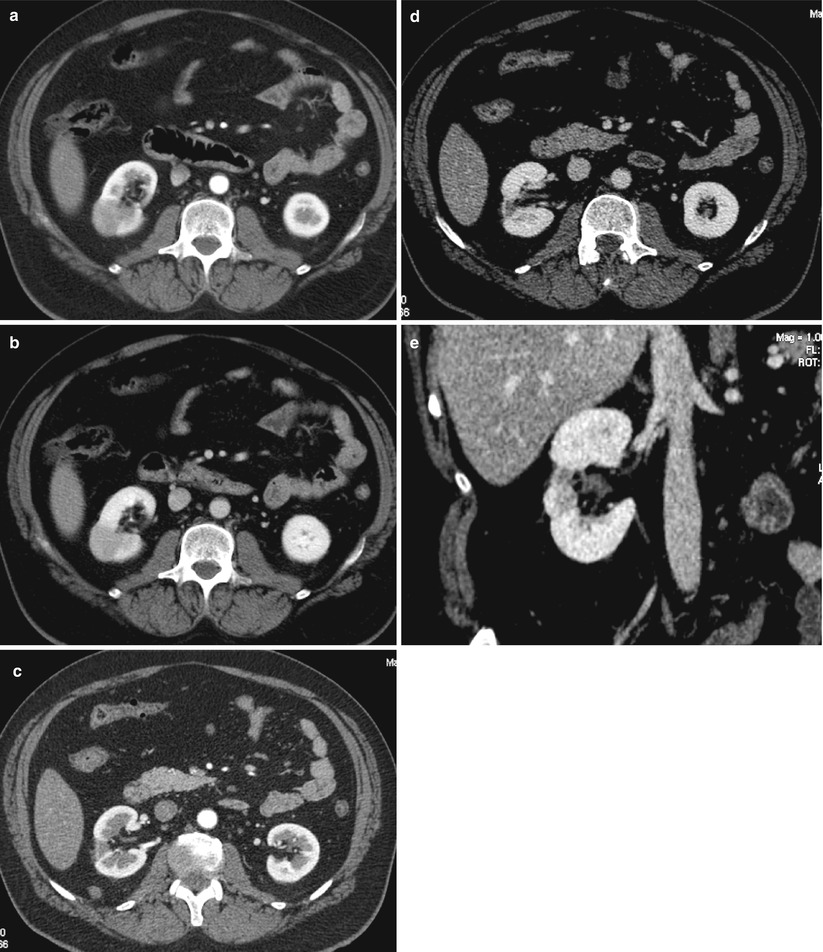

Fig. 6
Favorable evolution in a case of non-complicated acute focal pyelonephritis of the right kidney. Contrast-enhanced CT scans obtained during arterial (a) and nephrographic (b) phase show focal swelling with a round-shaped area of decreased attenuation radiating from the papilla to the posterior surface in the middle part of the right kidney. The 2-month follow-up shows the positive evolution of renal infection residuating with parenchymal volume reduction and renal scar, well demonstrated both in the arterial phase (c) and in the axial (d) and the coronal reconstructed (e) images of the nephrographic phase (Case courtesy of G. Regine, MD, S. Camillo Hospital, Rome, Italy)
The alternating bands of hyper- and hypoattenuation parallel to the axes of the tubules and collecting ducts, which characterize the so-called “striated nephrogram” typical of acute pyelonephritis, can be correlated to the obstructed tubules alternated to the normal tubules; stasis of the contrast material secondary to the obstruction of the collecting system is the reason for these striations, which have a lobar distribution, may be uni- or multifocal, and can be uni- or bilateral (Fig. 7). However, it is noteworthy that this CT finding is not pathognomonic of acute pyelonephritis and can also be seen in poorly hydrated patients and in normal kidneys when contrast agents with low osmolarity are used.
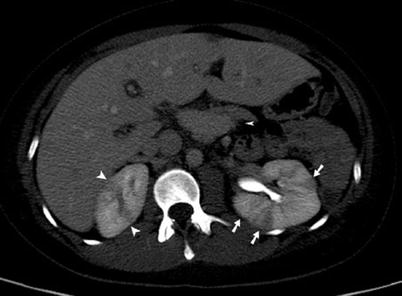

Fig. 7
Bilateral and multifocal acute bacterial pyelonephritis. The nephrographic phase of contrast-enhanced CT scan shows the typical striated pyelogram pattern in the left kidney (arrows) of an adult woman with bilateral acute bacterial pyelonephritis. Note also some focal wedge-shaped areas of the disease in the right kidney (arrowheads)
The finding of round, not wedge-shaped peripheral hypoattenuating renal lesions in the clinical setting of pyelonephritis should raise the suspect of hematogenous seeding (Fig. 8); nonetheless, when these lesions coalesce or involve the entire kidney, it may not be possible to distinguish between the ascending and hematogenous infection (Talner et al. 1994; Lee et al. 1980) (Fig. 9).
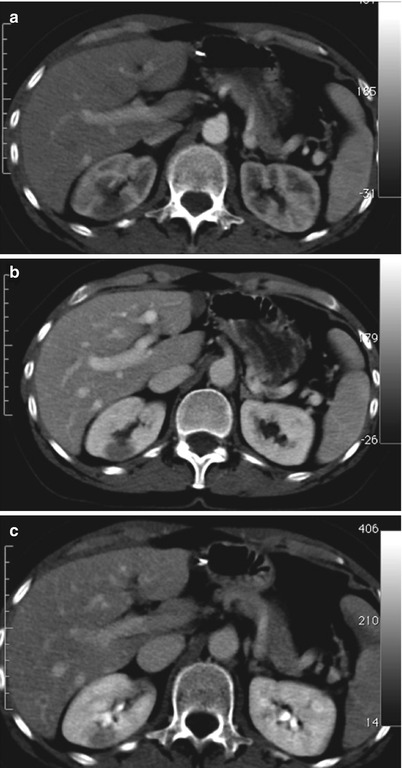
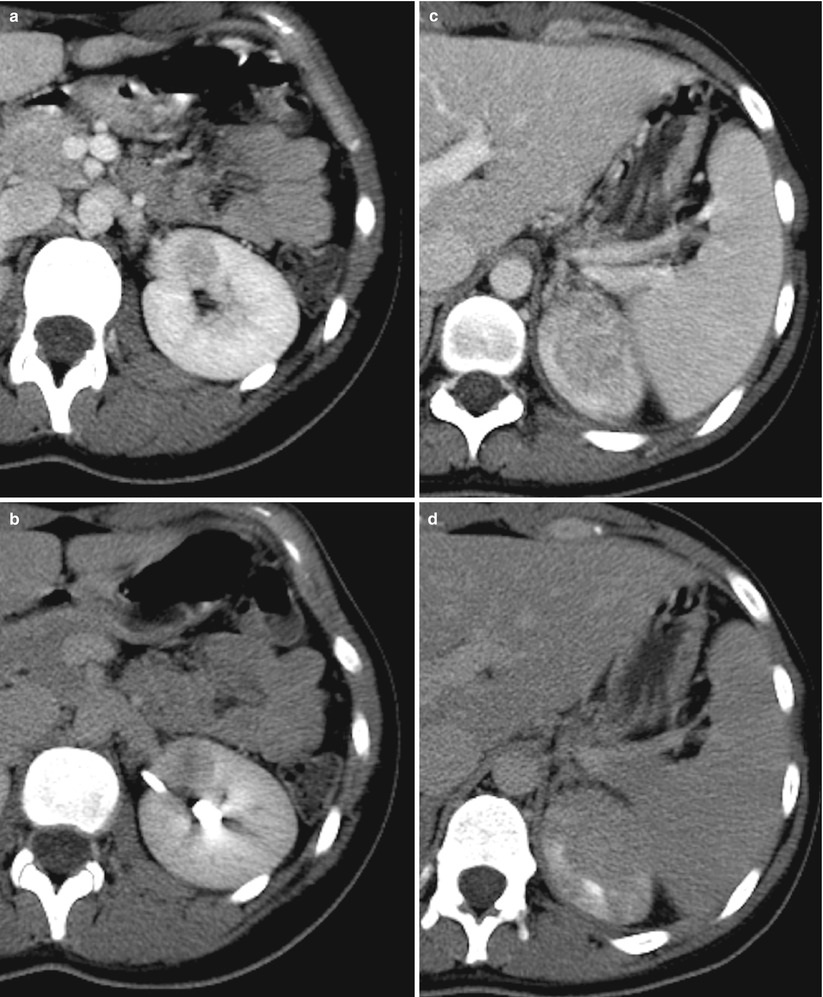

Fig. 8
Drug-abusing patient with clinical signs of acute pyelonephritis. Contrast-enhanced CT scans during arterial (a), nephrographic (b), and excretory (c) phases demonstrate the presence of a round, peripheral hypoattenuating lesion in the posterior upper half of the right kidney, suspected for hematogenous pyelonephritis

Fig. 9
Multifocal hematogenous pyelonephritis in a 50-year-old patient with bacterial endocarditis. Enhanced CT scans obtained during parenchymal nephrographic phase (a) and excretory phase (b) show the presence of a round peripheral hypoattenuating lesion in the anterior lip of the left kidney compatible with the diagnosis of hematogenous pyelonephritis. Contrast-enhanced CT scans obtained during nephrographic (c) and excretory (d) phases at the level of the upper pole of the kidney show a huge coalescent hypodense lesion not allowing differentiation between ascending and hematogenous infection (Case courtesy of M. Mandalà, MD, Hospital Cannizzaro, Catania, Italy)
According to Paick et al. (2013), CT can also be useful in grading parenchymal involvement in acute pyelonephritis, i.e., from grade 1 to grade 4, which strongly correlates with clinical severity and disease course.
Consequently, acute pyelonephritis grades, as determined by CT examination, have a good prognostic value, and it is valuable in predicting the clinical course of the disease.
Differential diagnosis for focal hypoattenuating areas includes a mass, areas of renal infarction, tumors, or scarring, in particular, when these CT findings are identified in the absence of clinically suspected pyelonephritis. The hypoattenuating areas caused by infarcts and tumors persist even after antibiotic treatment, whereas changes resolve following treatment when they are caused by pyelonephritis; moreover, scarring rarely occurs in adult patients. Furthermore, delayed CT scans can help differentiate tumor from inflammatory mass by showing persistent enhancement in areas of previously diminished enhancement (Fig. 4c).
CT commonly demonstrates secondary signs of renal infection and its complications. These signs include focal or global enlargement of the kidney, obliteration of the renal sinus and perinephric fat planes, calyceal effacement caused by the swelling of the adjacent affected renal parenchyma, pelvicalyceal wall thickening and enhancement secondary to ascending infections, soft tissue perinephric stranding, and thickening of the Gerota fascia (Goldman and Fishman 1991; Pickhardt et al. 2000; Soulen et al. 1989).
In particular, pelvicalyceal wall thickening and enhancement secondary to ascending infection may be the only imaging feature of acute pyelonephritis when CT is performed early, before renal damage is macroscopically evident with this imaging technique (Figs. 10 and 11).
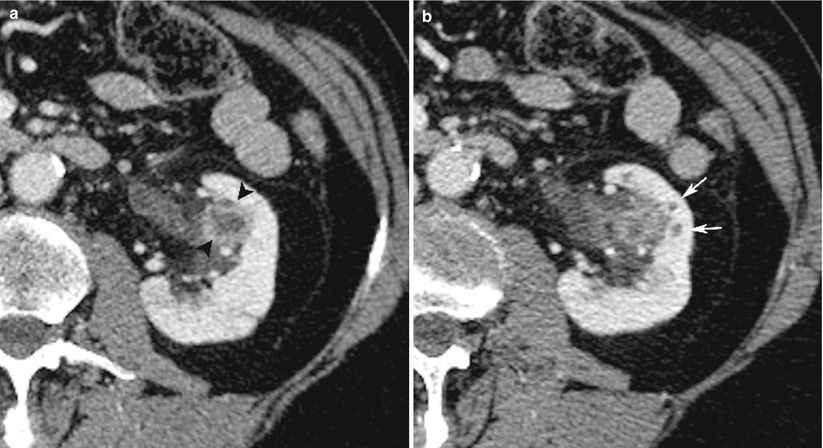
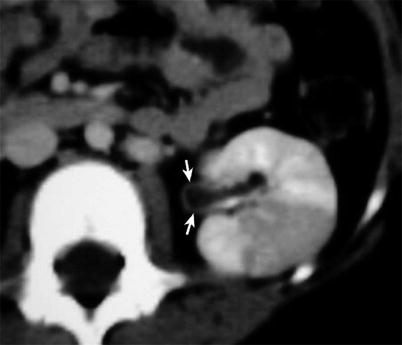

Fig. 10
Young male patient with left pyelitis. Contrast-enhanced CT scan (a) shows thickening and enhancement of the calyceal wall (arrowheads). A CT scan obtained at a lower level (b) demonstrates the presence of round, small areas of low density at the apex of the papillae (thin arrows) representative of early pyelonephritis with renal parenchyma involvement (Case courtesy of G. Regine, MD, S. Camillo Hospital, Rome, Italy)

Fig. 11
Diabetic patient with left kidney pyelitis and focal acute pyelonephritis. Mild thickness with faint enhancement of the pelvic wall suggesting acute pyelitis is well appreciable on the axial contrast-enhanced CT scan (arrows). Note also the huge wedge-shaped area of parenchymal involvement suggesting focal pyelonephritis (Case courtesy of V. Magnano San Lio, MD, Garibaldi Hospital, Catania, Italy)
It is important to observe that perinephric stranding alone should be interpreted with caution, because even in the presence of acute symptoms, it may relate to previous infections, trauma, or vascular disease (Urban and Fishman 2000).
Soft tissue filling defect in the collecting system may represent sloughed tissue from papillary necrosis, inflammatory debris, or blood clots.
Diffuse pyelonephritis is characterized by renal enlargement, poor enhancement, and poor excretion of contrast medium in proportion to the severity of infection (Fig. 12).
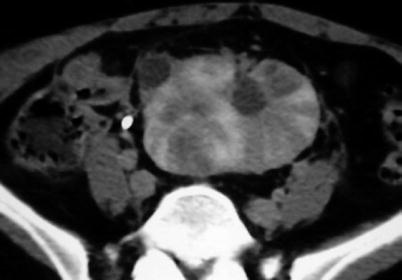

Fig. 12
Acute diffuse pyelonephritis in pelvic kidney. Contrast-enhanced CT scan reveals enlargement of the pelvic kidney, loss of contrast enhancement with multifocal regions of diminished enhancement radiating from the papillae to the cortical surface. Note also a small exophytic simple renal cyst
Finally, it also has to be noted that acute pyelonephritis is often a dynamic process in which the areas of the kidney characterized by improvement can be adjacent to the areas of progression.
1.2.4 Nuclear Medicine
As far as NM examinations are concerned, the use of dimercaptosuccinic acid (DMSA) should be considered for the detection of acute pyelonephritis and renal scarring (Eggli and Tulchinsky 1993; Rossleigh 2009). Cortical scintigraphy, indeed, has shown superior sensitivity in detecting pyelonephritis and renal scars compared with IVU or renal ultrasonography (Eggli and Tulchinsky 1993; Bjorgvinsson et al. 1991; Hatch and Ouwenga 2006).
The uptake of 99mTc-DMSA is related to the relative amount of functioning renal tissue and does not measure a specific localization process. The exact mechanism by which it localizes in the kidney is unclear although glomerular filtration, tubular secretion, and tubular resorption have been suggested to play a role; it seems that the tracer is taken up by the peritubular vessels. Approximately 40–50 % of 99mTc-DMSA accumulate in the cortical tubules within 1 h and remain for 24 h. Images (at least posterior and oblique posterior views) are obtained 2–3 h after injection, with high-resolution collimators (Piepsz et al. 2001 ). The examination delivers a significant radiation dose to the patient (3.15R to the kidneys and 4.25R to the renal cortices for a 185-MBq injected dose) (Hatch and Ouwenga 2006; Piepsz et al. 1999).
The normal DMSA scan demonstrates nearly equal distribution of radiopharmaceutical throughout the cortex (the outer part corresponding to the cortex is more active than the inner part, the medulla and the collecting system); the contours of the kidney are generally round although a contour can be flat without suggesting a lesion. Moreover, the kidney may appear triangular or “pear shaped”. Kidney rotation and/or the normal contrast of the hypoactive poles with the hyperactive columns of Bertin may cause false interpretation of the images (Piepsz et al. 1999).
There is no consensus on the use of cortical scintigraphy in acute renal infections (Hatch and Ouwenga 2006; Piepsz et al. 1999). Several authors suggest that acute renal scintigraphy is not necessary because half of the acute lesions are transitory and disappear (Stokland et al. 1996); others consider that clinical and biological arguments constitute a weak evidence for acute pyelonephritis and that patients with lack of clinical and biological signs or with negative or equivocal urine cultures may still have UTI with obvious acute renal lesions (Conway and Cohn 1994). Only 58 % of experts in one consensus study (Piepsz et al. 1999) were using a cortical imaging study during the acute phase of an infection; among those who perform acute DMSA, 22 % did it for any first UTI, 22 % for any UTI, and 56 % only in case of high suspicion of acute pyelonephritis. There is consensus that acute DMSA should be performed without delay (3–7 days).
Pyelonephritic lesions appear as focal, multiple, or diffuse reduction in the accumulation of the radiopharmaceutical (probably because of local ischemic changes in the infected segments of renal parenchyma) without cortical or volume loss; renal cortical contour remains intact (Fig. 13).
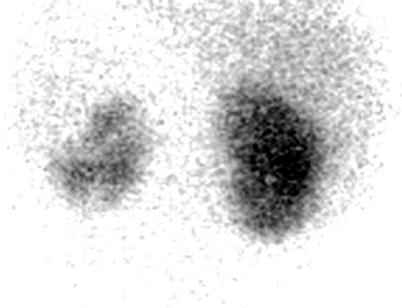

Fig. 13
99mTc-DMSA scintigraphy (posterior view) in a patient with suspected acute pyelonephritis shows reduced accumulation of the radiopharmaceutical in the left kidney mainly involving the upper pole. The relative left and right dimercaptosuccinic acid (DMSA) uptakes were 32 and 68 %, respectively. Ultrasonography (not shown) was negative
It has been demonstrated on animal models that the presence of a scintigraphic abnormality strongly correlates with the extension of the anatomic lesion. In the clinical practice, however, scintigraphic abnormalities in case of acute UTI are not specific unless any other underlying disease such as renal abscess, hydronephrosis, cysts, or complicated duplex kidney has been excluded. It is mandatory, therefore, to combine scintigraphy with ultrasound which allows differentiation between these situations.
Many reports suggest that the diagnostic confidence of the NM examination can be increased by adding either pinhole or SPECT imaging to the planar studies (Piepsz et al. 1999 ; Rossleigh 2009).
Determination of the relative left and right DMSA uptake is generally performed (on the posterior view with or without lateral view for the correction of kidney depth; one can also determine the geometric mean using both the anterior and posterior views; no consensus is reached whether or not the background should be subtracted). The normal lowest value for relative uptake is approximately 45 %. The normal side to side distribution can completely miss bilateral small kidney; inversely, the normal unilateral duplex situation can be associated with abnormally high relative function. For some authors, a percentage below 45 % during acute UTI predicts permanent damage (Piepsz et al. 1999; De Sadeleer et al. 1993). Hydronephrosis is not a contraindication for a DMSA scintigraphy, but constitutes a pitfall giving rise to the overestimation of the relative function on the side of obstruction because of the accumulation of the excreted radiotracer; in such cases, late images (4–24 h) or furosemide injection may be helpful (Piepsz et al. 2001).
There is a wide consensus among NM physicians for a systematic use of cortical scintigraphy in the detection of sequelae, namely, renal scarring after an episode of pyelonephritis, whatever the number of previous infections; there is no consensus concerning the adequate moment for the detection of sequelae (3–6–12 months); however, most of the authors suggest that DMSA examination should not be performed until at least 6 months after acute infection (Hatch and Ouwenga 2006; Piepsz et al. 1999; Stokland et al. 1999; Sixt and Stokland 1998; Ghasemi et al. 2013; Supavekin et al. 2013).
Renal parenchymal scintigraphy has demonstrated that the scars (which appear as defects in uptake associated with cortical thinning and volume loss resulting in a localized deformity of the renal outline) occur at the same site as the region in which pyelonephritis is detected. The best predictor of renal sequelae is probably the presence of a renal abnormality during the acute phase of infection. Therefore, the risk of developing persistent scars is low when early scintigraphy is normal, and this may influence further strategy. Many argue that if the DMSA study is normal at the time of acute UTI, no further investigation is required, because the kidneys have not been involved and thus there will be no late sequelae. Others use the acute DMSA study to determine the intensity of antibiotic therapy (Rossleigh 2007).
1.2.5 MRI
MRI is not normally employed in patients suffering from acute renal infections.
Nevertheless, MRI is an emerging, strong modality for the evaluation of kidney and urinary tract pathology, including acute and chronic renal infections, and it may eventually prove of high value when utilized appropriately and in selected cases.
Renal MRI potentially offers a cost-effective and radiation-free alternative in the radiologic diagnosis of acute pyelonephritis. Due to the lack of ionizing radiation, it is especially useful in patients for whom exposure to X-rays should be avoided (e.g., pregnant women or children). Moreover, it should be used in patients with allergy to iodinated contrast agents.
Conventional MRI findings reproduce the pathologic change that characterizes acute pyelonephritis, with signal changes reflecting the distribution of edema within the renal parenchyma.
The kidney can show focal or diffuse enlargement with the affected inflammatory areas following the expected signal intensity of edematous lesions, i.e., low signal intensity on T1-weighted images and high intensity on T2-weighted images, with loss of normal corticomedullary differentiation (Craig et al. 2008; Stunel et al. 2007). The edematous inflammatory areas are better depicted using T2-weighted images with fat suppression (FS) technique (Fig. 14).
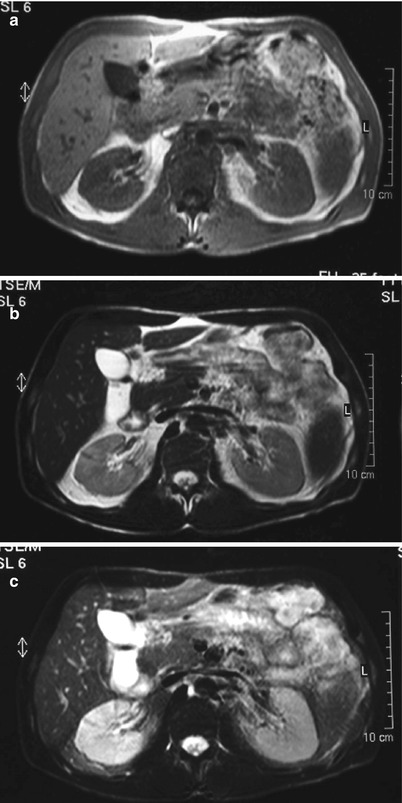

Fig. 14
Conventional MRI in a young woman with right acute bacterial pyelonephritis. (a) Gradient-echo T1-weighted axial magnetic resonance (MR) image passing through the middle part of both the kidneys does not show any difference in size and signal intensity between the right and left kidney. (b) Axial T2-weighted fast spin-echo image shows a faint and diffuse high signal intensity of right renal parenchyma. (c) Axial T2-weighted fast spin-echo image with fat suppression (FS). The high intensity of the affected renal parenchyma, due to diffuse edema, is highlighted and well recognizable (Case courtesy of G. Cardone, MD, S. Raffaele Hospital, Milan, Italy)
Perirenal stranding or small amount of perinephric fluid can be seen, but these are aspecific signs found in several other renal and perirenal pathologic conditions.
MRI with gadolinium is very helpful in exactly depicting areas of renal parenchyma involvement. The MRI features are quite similar to those of CT scan, with focal or diffuse, round, or wedge-shaped area of decreased enhancement compared with the normal enhancement of the not-involved neighboring renal parenchyma (Fig. 15).
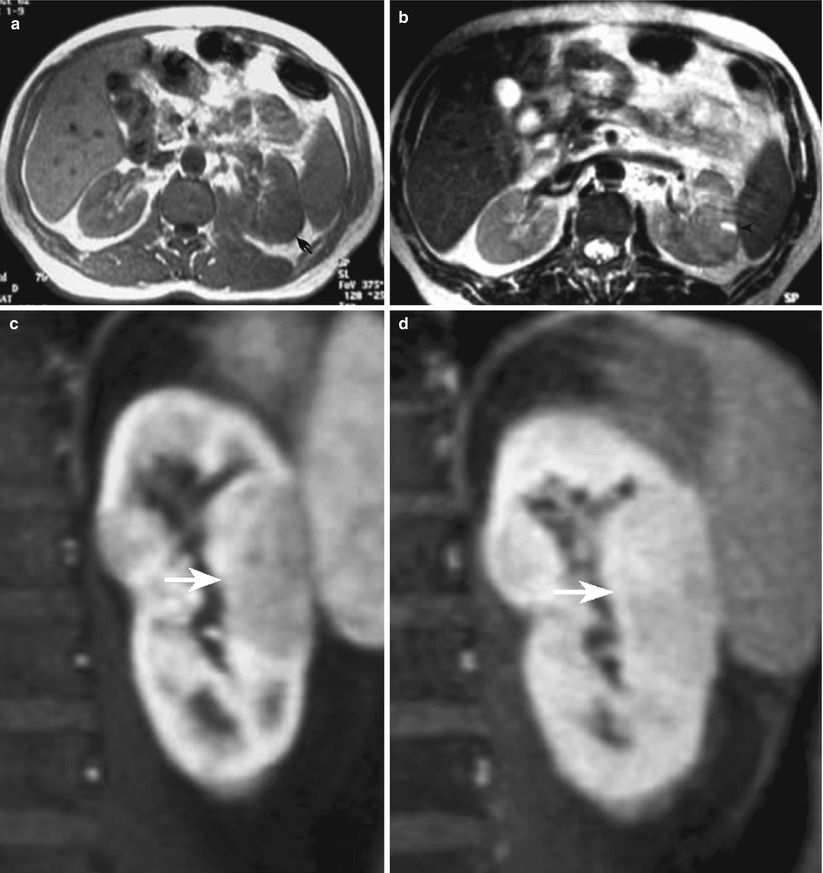

Fig. 15
Patient with acute focal bacterial pyelonephritis of the left kidney. (a) Gradient-echo axial T1-weighted MR image passing through the middle part of the kidney. Mild enlargement of left kidney with focal swelling of the posterior middle portion (arrow). (b) Axial T2-weighted fast spin-echo image shows round mild hyperintense area in the posterior part of the left kidney; a small preexisting hyperintense cyst compressed from the involved parenchyma is shown (arrowhead). (c, d) Contrast-enhanced T1-weighted gradient-echo coronal images. The involved renal parenchyma by the acute bacterial inflammation is well appreciable both in the arterial (c) and in the nephrographic phase (d) of the study as a peripheral hypointense wedge-shaped area (arrow) (Case courtesy of M. Scialpi, MD, University of Perugia, Italy)
In order to increase the conspicuity of intrarenal lesion, Lonergan et al. (1998) proposed an alternative MRI protocol in a series of children aged 2–16 years suffering from acute pyelonephritis.
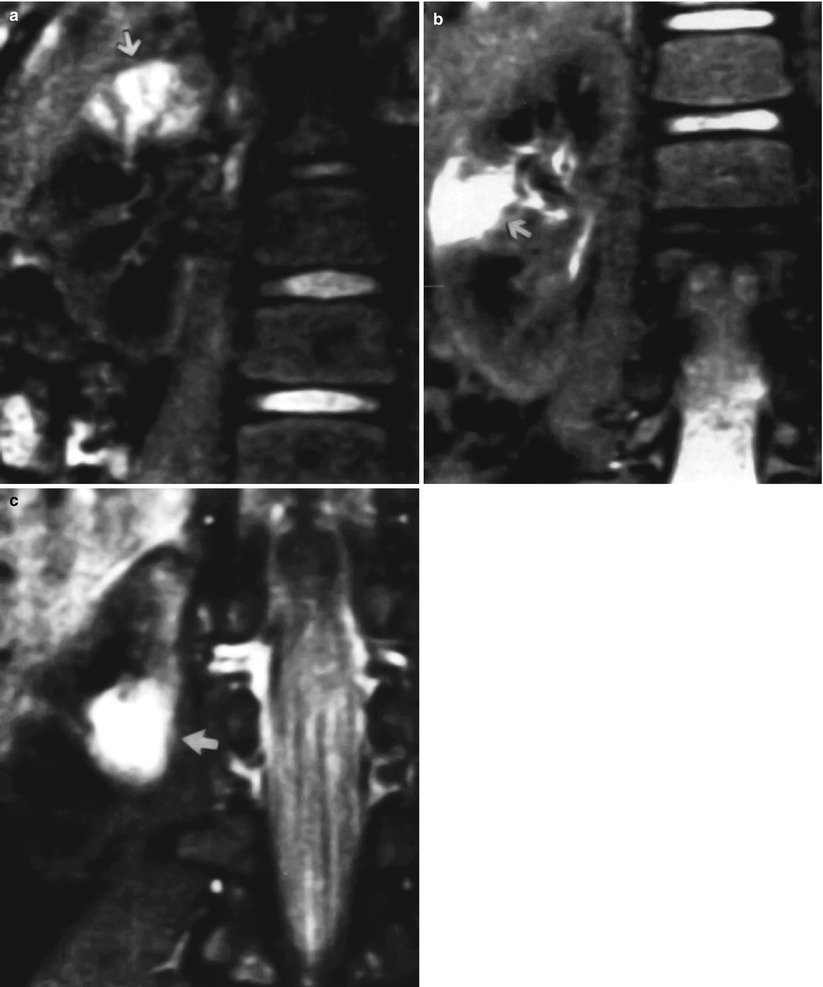
In fact, using a gadolinium-enhanced inversion-recovery MR imaging, it is possible to use the negative enhancement effect of the paramagnetic contrast agent. The normally well-perfused renal parenchyma becomes hypointense or dark, because gadolinium decreases the signal intensity on inversion-recovery images (“contrast negative effect”); conversely the less well-perfused areas of acute inflammation remain high in signal intensity, due to the concomitant effects of edema and poor delivery and concentration of gadolinium (Figs. 16 and 17).
Get Clinical Tree app for offline access

Fig. 16
(a–c) Young patient with multifocal acute bacterial pyelonephritis. Coronal gadolinium-enhanced inversion-recovery MR images (2,000/16/160, 90° flip angle) at three different levels show foci of high signal intensity (arrows




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree