Renal Inflammatory Disease
Inflammatory conditions involving the urinary tract are among the most common infectious disorders affecting humans. In most cases, the infection is confined to the lower urinary tract, and the diagnosis is established by clinical or laboratory studies. Imaging studies are not required when there is prompt resolution after appropriate therapy. However, when the kidney is involved by the inflammatory process, or when the precise diagnosis is not known, renal imaging studies may play an important role in the diagnosis and management.
Pathologically, inflammatory disease involving the kidney can be divided into two broad groups: (1) glomerulonephritis, which involves an immunologic injury of the glomerulus, and (2) interstitial nephritis, which results from the effect of an infectious or toxic agent on the renal parenchyma. Radiologic studies play a limited role in the diagnosis and management of glomerulonephritis (see Chapter 11). Interstitial nephritis is divided into two major subgroups: (1) noninfectious interstitial nephritis, which is usually caused by the action of toxic agents on the kidney, and (2) infectious interstitial nephritis, which is the result of the action of a pathogen. In most cases, infectious interstitial nephritis is caused by a bacterial organism; in such cases, the disease is called acute pyelonephritis.
▪ BACTERIAL INFECTIONS
Pathophysiology
Bacteria usually reach the kidney through the ureter as a result of ascending infection from the lower urinary tract. In children, this commonly occurs as a result of vesicoureteral reflux; in adults, however, frank reflux is uncommon, and bacteria are thought to ascend to the kidney through the ureter against the antegrade flow of urine. Some bacteria, for example, Escherichia coli, have demonstrated the ability to elaborate a protein termed P fimbriae that facilitate bacterial adhesion to the cells of the urothelium.
Women have a much higher incidence of lower urinary tract infection because of the short length of the female urethra. In patients younger than 50 years, bacterial infections of the kidney are much more common in women than in men. Beyond this age, however, the incidence of urinary tract infection in men increases, as a result of urinary stasis caused by benign prostatic hypertrophy. Much less commonly, bacterial infections are spread to the kidney hematogenously.
Patients often present with fever, flank pain, chills, and other systemic symptoms such as nausea, vomiting, and malaise. These symptoms occur in patients with upper urinary tract infection and help to differentiate them from those infections involving only the lower urinary tract. Conditions that predispose patients with lower urinary tract infection to renal involvement include vesicoureteral reflux, urinary tract obstruction, calculi, altered bladder function, altered host resistance, pregnancy, and congenital urinary tract anomalies. There is usually a prompt response to appropriate antibiotic therapy, and diagnostic imaging studies are seldom necessary. Immunocompromised patients and those with underlying diabetes mellitus are of particular concern because they are more vulnerable to the development of complications from acute pyelonephritis. It is also more difficult to establish the diagnosis on clinical grounds in patients with diabetes; as many as 50% of these patients will not present with the typical flank tenderness that helps differentiate pyelonephritis from lower urinary tract infection. Hence, a more aggressive approach to imaging in patients with diabetes is indicated. Gram-negative enteric pathogens, including E. coli, Proteus mirabilis, Pseudomonas aeruginosa, and Klebsiella spp., are responsible for the vast majority of bacterial renal infections. In the preantibiotic era, gram-positive urine pathogens including Staphylococcus and Streptococcus spp. were relatively common; however, currently they cause only a small minority of infections.
Acute pyelonephritis is an acute bacterial infection of the kidney manifested by infiltration of the renal interstitium with
neutrophils. The kidney is swollen; the tissues are hyperemic; and small microabscesses, 1 to 5 mm in diameter, may be present. The process may be unilateral or bilateral, and focal or widespread; its distribution is usually focal, so that involved areas are interspersed with zones of unaffected renal tissue. Although most cases of renal infection produce changes in the renal parenchyma, occasionally just the walls of the ureter and collecting system are involved, producing edema, inflammation, and radiologically visible thickening. Uncommonly, there may be coalescence of the small microabscesses in acute pyelonephritis with underlying tissue liquefaction, so that an acute renal abscess is formed. After formation of the abscess, fibroblasts migrate into the area of inflammation to build a wall between the normal parenchyma and the necrotic tissue. This “walling-off” process is characteristic of chronic abscesses, and the remainder of the renal parenchyma returns to normal. If the abscess breaks through the renal capsule, a perinephric abscess is formed. In patients with pyelonephritis and ureteral obstruction sufficiently severe that the affected kidney is oliguric or anuric, the collecting system becomes filled with pus. If pyelosinus extravasation should occur, a perinephric abscess may be formed by this mechanism as well.
neutrophils. The kidney is swollen; the tissues are hyperemic; and small microabscesses, 1 to 5 mm in diameter, may be present. The process may be unilateral or bilateral, and focal or widespread; its distribution is usually focal, so that involved areas are interspersed with zones of unaffected renal tissue. Although most cases of renal infection produce changes in the renal parenchyma, occasionally just the walls of the ureter and collecting system are involved, producing edema, inflammation, and radiologically visible thickening. Uncommonly, there may be coalescence of the small microabscesses in acute pyelonephritis with underlying tissue liquefaction, so that an acute renal abscess is formed. After formation of the abscess, fibroblasts migrate into the area of inflammation to build a wall between the normal parenchyma and the necrotic tissue. This “walling-off” process is characteristic of chronic abscesses, and the remainder of the renal parenchyma returns to normal. If the abscess breaks through the renal capsule, a perinephric abscess is formed. In patients with pyelonephritis and ureteral obstruction sufficiently severe that the affected kidney is oliguric or anuric, the collecting system becomes filled with pus. If pyelosinus extravasation should occur, a perinephric abscess may be formed by this mechanism as well.
Imaging Approach to Renal Inflammatory Disease
The rationale for performing an imaging study is not to diagnose acute pyelonephritis, but to look for an underlying anatomic abnormality that may have predisposed the patient to the infection, to search for a calculus or an obstruction that may prevent a rapid therapeutic response, or to diagnose a complication of the infection such as a renal or perinephric abscess. If the investigation is confined to those patients who do not become afebrile after 72 hours of appropriate antibiotic therapy, the proportion of patients with findings that have immediate clinical significance increases significantly.
Excretory urography has been almost entirely abandoned for imaging patients with urinary tract infection. Contrast-enhanced computed tomography (CT) or CT urography (CTU) is the imaging study of choice for the diagnosis of atypical pyelonephritis or to look for a potential complication of the infection such as a renal or perinephric abscess, or renal emphysema.
Conventional gray-scale ultrasound detects most instances of abscess, obstruction and stones in infected patients, and sometimes can suggest pyonephrosis by detecting small particles in infected urine, but may fail to detect these abnormalities if they are minor or if the patient is difficult to examine. Ultrasound is less sensitive than CT in revealing foci of inflamed renal parenchyma. However, ultrasound is safe, is relatively inexpensive, and can diagnose most of the abnormalities that require specific treatment. Thus, it remains a commonly used modality for patients with renal infection.
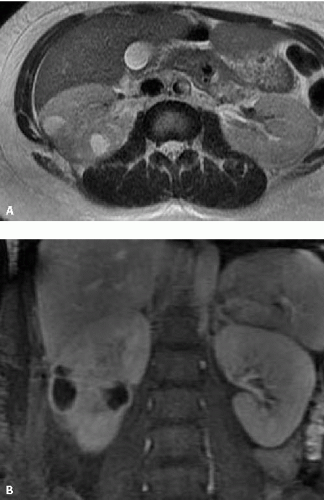
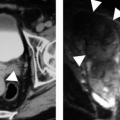
Various other imaging studies are of value in selected patients. Magnetic resonance imaging (MRI) may demonstrate infected regions of the renal parenchyma to be mildly hyperintense on T1-weighted images after gadolinium enhancement, and can demonstrate obstruction and abscesses, but often fails to reveal small stones; the technique should probably be reserved for problem solving in patients who cannot be given iodinated contrast material (Fig. 9.1). Retrograde pyelography is of value in patients with severe infection and obstruction that cannot be demonstrated noninvasively. Antegrade pyelography can be used as an alternative to the retrograde study if there is hydronephrosis. Retrograde and antegrade pyelography may serve both as diagnostic and planning maneuvers in patients with obstructive pyonephrosis who are to be treated with ureteral stents or percutaneous nephrostomy tubes, respectively. Voiding cystourethrography is used to demonstrate vesicoureteral reflux, but is performed only rarely in adults.
In children with multiple urinary infections, the classic imaging approach involves searching for reflux and other bladder and urethral abnormalities with voiding cystourethrography; demonstration of reflux often leads to a search for renal infection and scars with renal scintigraphy (usually with technetium-99m-dimercaptosuccinic acid [99mTc-DMSA]) and/or renal gray-scale and Doppler ultrasound. Recently, there has been a growing tendency to use a “topdown” approach to imaging, in which renal abnormalities are sought with ultrasound or scintigraphy, and efforts to diagnose reflux are reserved for patients with renal findings that suggest it.
Acute Pyelonephritis
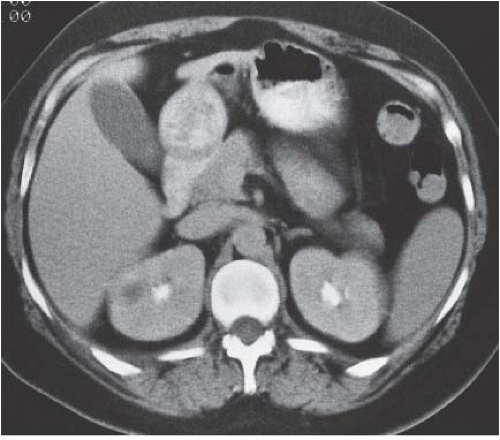
Acute uncomplicated pyelonephritis is the most common bacterial infection involving the kidney. The infection usually responds quickly (within 48 to 72 hours) to antibiotic therapy, and generally does not lead to permanent morphologic damage. Excretory urography is usually normal, but findings may include (1) diffuse renal enlargement, reflecting the edema that accompanies the infection; (2) delay in the appearance, and diminution of concentration of the contrast medium in the renal collecting system; (3) attenuation of the intrarenal collecting system, as the result of diminished excretion and parenchymal edema; and (4) a decrease in the density of the nephrogram in the affected portion of the kidney. In general, the degree of radiographic abnormality and the degree of impairment of contrast excretion reflect the severity of the interstitial inflammatory disease (Fig. 9.2).
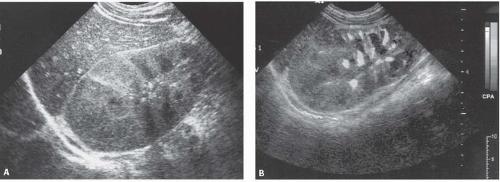
Ultrasound in patients with acute uncomplicated pyelonephritis may be normal or shows diffuse or focal renal enlargement with regions of increased or decreased echogenicity of the renal parenchyma (Fig. 9.3). The normal corticomedullary differentiation may be lost.
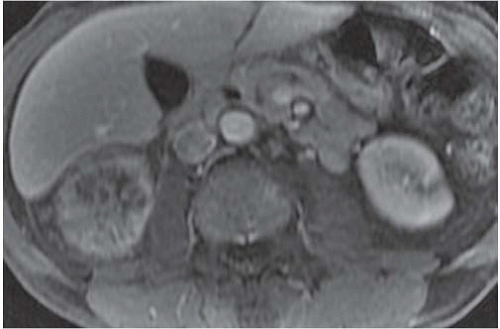
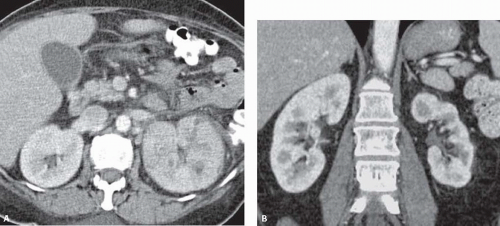
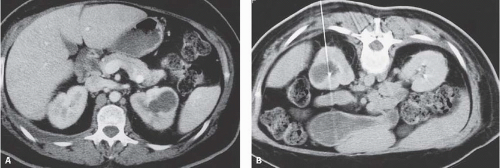
The most complete information regarding the nature and extent of the inflammatory process is provided by CT. Unenhanced scans may be normal or show only renal enlargement; there may be perinephric stranding, which reflects inflammation or edema. After intravenous contrast administration, areas of decreased contrast enhancement appear (Fig. 9.4). These regions may be inhomogeneous or striated and wedge-shaped or rounded, and may almost always extend to the renal capsule (Fig. 9.5). Their distribution is variable; they may be solitary or multiple and unilateral or bilateral, and may involve little or most of the parenchyma. Their distribution is typically patchy; diffuse homogeneous involvement of the renal parenchyma is rare and is likely to appear only when there is ureteral obstruction. If delayed scans are obtained, the abnormal
areas may reveal a dense nephrogram, which persists after the normal parenchyma has lost its enhancement (Fig. 9.6). Differential diagnosis of regions of diminished or inhomogeneous enhancement may include acute ischemia or contusion; if there is focal swelling, the lesion may resemble an infiltrating tumor. Clinical data often permit a specific diagnosis, and ischemia may be accompanied by the peripheral rim sign or visible vascular abnormalities.
areas may reveal a dense nephrogram, which persists after the normal parenchyma has lost its enhancement (Fig. 9.6). Differential diagnosis of regions of diminished or inhomogeneous enhancement may include acute ischemia or contusion; if there is focal swelling, the lesion may resemble an infiltrating tumor. Clinical data often permit a specific diagnosis, and ischemia may be accompanied by the peripheral rim sign or visible vascular abnormalities.
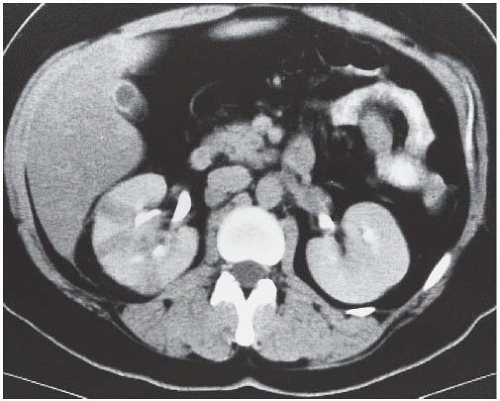 FIGURE 9.4. CT appearance of acute pyelonephritis. Relatively narrow-striated zones of decreased contrast enhancement are present in the right renal parenchyma. |
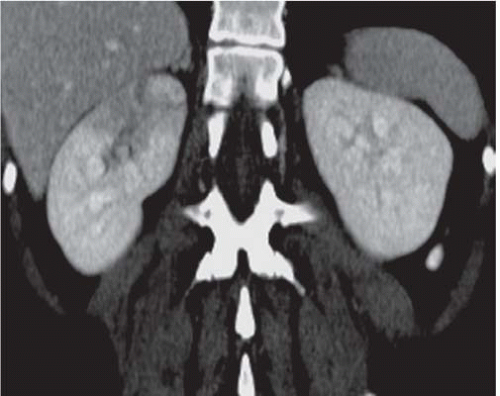 FIGURE 9.5. CT urogram of acute pyelonephritis. The superior portion of the right kidney has a diminished and inhomogeneous nephrogram. |
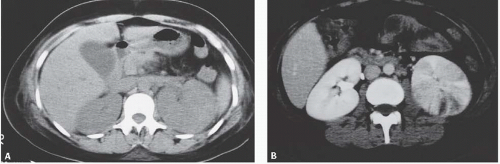
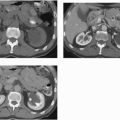
The appearance of pyelonephritis on CT may be modified by antibiotic therapy. Partially treated patients may demonstrate a rounded or ovoid area of decreased enhancement with poorly defined margins (Fig. 9.7). Long-term follow-up studies in patients with mild disease may show a return to normal renal morphology and enhancement (Fig. 9.8). With more severe disease, there may be generalized wasting of the kidney or focal atrophy (Fig. 9.9) and focal calyceal clubbing, suggestive of papillary necrosis. The etiology of these morphologic changes has been postulated to involve ischemic insult to the kidney as a result of the inflammatory process (Fig. 9.10). Scarring from renal infection had been thought to be a feature of childhood pyelonephritis; however, cross-sectional imaging studies have demonstrated that scarring may occur
in adults after severe infections. Radionuclide imaging in patients with acute pyelonephritis has been reported using renal cortical imaging agents, such as 99mTc-DMSA, and agents, such as gallium 67(67Ga) citrate, which image areas of inflammation. Renal cortical imaging studies may show an inhomogeneous distribution of the radionuclide within the affected kidney or polar defects with asymmetric tracer uptake. In some cases, a pattern that was specific for the diagnosis of pyelonephritis, termed the flare pattern, representing a striate distribution of decreased radioactivity was present.
in adults after severe infections. Radionuclide imaging in patients with acute pyelonephritis has been reported using renal cortical imaging agents, such as 99mTc-DMSA, and agents, such as gallium 67(67Ga) citrate, which image areas of inflammation. Renal cortical imaging studies may show an inhomogeneous distribution of the radionuclide within the affected kidney or polar defects with asymmetric tracer uptake. In some cases, a pattern that was specific for the diagnosis of pyelonephritis, termed the flare pattern, representing a striate distribution of decreased radioactivity was present.
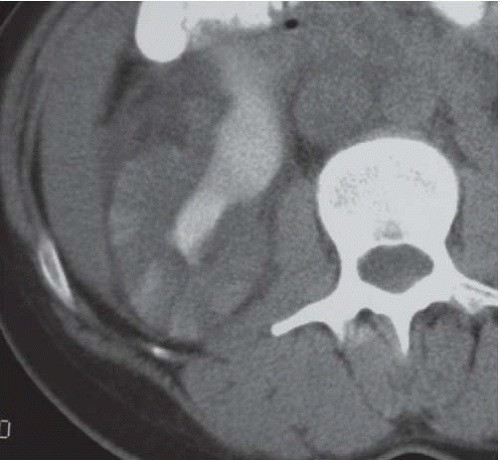 FIGURE 9.6. Acute pyelonephritis; delayed CT. The abnormal regions are revealed by wedge-shaped regions of retained parenchymal contrast. |
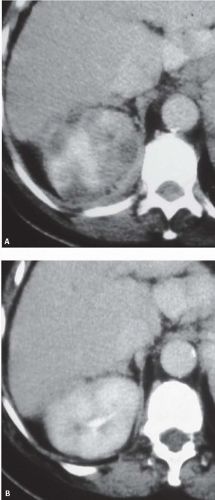 FIGURE 9.8. Acute pyelonephritis. A: Before treatment, the right kidney is severely affected, revealing regions of very poor enhancement. B: After treatment, morphology and enhancement are normal. |
Acute Renal Abscess
Most renal abscesses form as a result of the coalescence of small microabscesses that are present in acute pyelonephritis. The predominant organisms responsible for abscesses are gram-negative enteric species that occur in the setting of diabetes mellitus, drug abuse, vesicoureteral reflux, or renal calculus disease. Acute abscesses may be solitary or may form simultaneously in multiple locations in the kidney. Multiple lesions are less common and suggest hematogenous dissemination.
COMPUTED TOMOGRAPHIC FINDINGS IN ACUTE PYELONEPHRITIS
Renal enlargement
Focal bulge in renal contour
Wedge-shaped or striated zones of decreased contrast enhancement
Perinephric stranding or edema
The signs and symptoms of an acute renal abscess are difficult to distinguish from those of pyelonephritis; the diseases often coexist. Fever, leukocytosis, pyuria, and flank pain are common. There is frequently a history of prior antibiotic therapy with recrudescence of symptoms on cessation of treatment. Renal abscesses appear as a continuum of conditions. Acute pyelonephritis may progress to a cluster of small abscesses, which may or may not coalesce, so that there may be a single fluid-filled cavity, a multiloculated cavity, or a phlegmonous region in which a network of solid tissue planes separate multiple small abscesses.
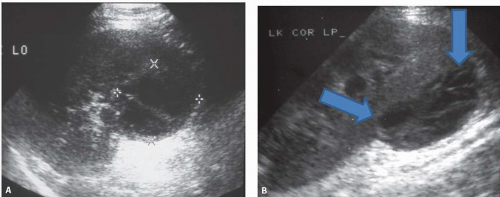
On sonography, abscesses appear as relatively sonolucent lesions with differing amounts of solid tissue echoes; the pus may contain lowamplitude echoes (Fig. 9.10). There is usually enhanced through-transmission of the ultrasound beam. Doppler studies show no flow in the necrotic pus-filled regions; the amount of flow in the wall is variable.
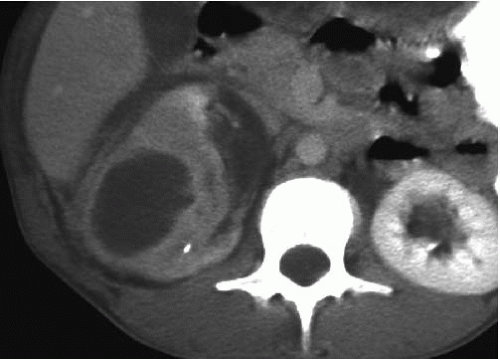
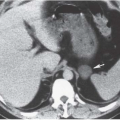
CT is the imaging study of choice for the diagnosis of an acute renal abscess. The lesion is a low-attenuation (10 to 20 HU) rounded or ovoid mass that does not enhance with contrast administration (Fig. 9.11). As a result of the surrounding inflammatory process, the borders of the mass are usually indistinct; the degree of enhancement of the abscess wall is variable. The presence of gas within the abscess is virtually pathognomonic of an abscess. There is usually thickening of Gerota fascia, and increased density may be found in the adjacent perinephric and mesenteric fat. The abscess may or may not extend into the perinephric space. MR findings reflect those seen on CT. The center of the abscess has the characteristics of nonenhancing fluid, and the periphery of the lesion may be of varying thickness and enhance to varying degrees (Fig. 9.12). Radionuclide scanning with technetium-labeled DMSA is occasionally used, but has largely been replaced by CT.
Chronic Renal Abscess
When fibroblasts migrate into the area of an acute renal abscess and form a barrier between the abscess and the remainder of the kidney, a chronic renal abscess is formed. This may result in a transition zone of inflammatory tissue between the liquid center of the abscess and the normal renal parenchyma (Fig. 9.13).
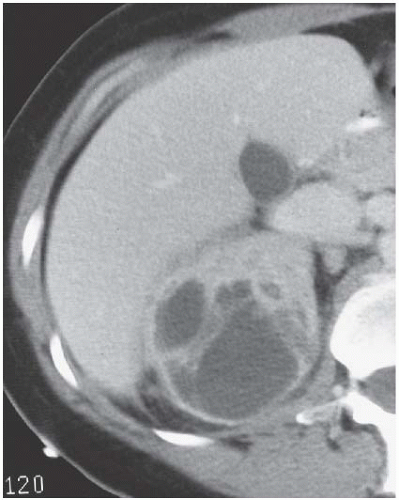 FIGURE 9.11. Acute renal abscess. An unenhanced CT scan demonstrates an acute multiloculated renal abscess. |
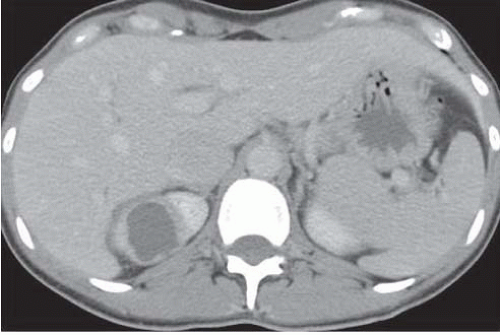 FIGURE 9.13. Renal abscess. There is a zone of inflammatory tissue between the liquid center and the normal renal parenchyma in the right kidney. |
On imaging studies, a chronic renal abscess is seen as a fluidfilled mass. Although the interior of the mass is avascular, there is frequently a prominent enhancing rim seen on CT. Although this rim is characteristic of a chronic renal abscess, it may also be found in some necrotic or cystic renal neoplasms. On ultrasonography, chronic renal abscesses appear as complex intrarenal masses or fluid collections. In some instances, the rim of granulation tissue may be identified; however, ultrasonography has not proven to be as reliable as CT in identifying the nature of the lesion.
Perinephric Abscess
A primary perinephric abscess forms when an intrarenal abscess breaks through the renal capsule into the perinephric space or as a result of obstruction with extravasation of infected urine. Secondary perinephric abscesses may form when infection is spread to the perinephric space hematogenously from an external source. A perinephric abscess may form from acute inflammation of an adjacent organ or from perforation of adjacent gut, that is, ruptured appendix or diverticulitis.
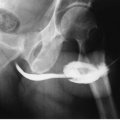
The signs and symptoms of a perinephric abscess are nonspecific. In most cases, symptoms of urinary infection have been present for periods longer than 2 weeks. Fever is usually intermittent and low grade. As many as 25% of patients have normal urinalysis. The development of a perinephric abscess as a complication of renal inflammatory disease is more common in patients with large staghorn calculi (Fig. 9.14), pyonephrosis, diabetes mellitus, or a neurogenic bladder.
On radiographs, large perinephric abscesses may be identified as soft-tissue masses in the perinephric space. The psoas margin may be obscured. Air secondary to gas-forming organisms is found in a number of large perinephric abscesses.
On ultrasonography, perinephric abscesses appear as masses of variable echogenicity adjacent to the kidney. Gas within the abscess will demonstrate acoustic shadowing, but when the abscess is anterior to the kidney, gas may be confused with intestinal gas.
The imaging study of choice for the detection of perinephric abscess is CT. Such abscesses are best detected when contrast-enhanced scans are obtained, but the abnormality is usually visible even on unenhanced studies. The strength of CT is its ability to define precisely the boundaries of the process, so that extension into the psoas muscle (Fig. 9.15), the posterior pararenal space, and the true pelvis may all be accurately detected. Like an intrarenal abscess, a perinephric abscess may demonstrate an enhancing rim on CT or MR (Fig. 9.16).
Percutaneous Drainage of Renal and Perinephric Abscess
Percutaneous drainage of renal and perinephric abscesses using radiologic guidance is the preferred method of therapy if the lesion is large; small renal abscesses may be successfully treated by intravenous antibiotics alone. Percutaneous drainage provides satisfactory clinical results using only local anesthesia and obviates the need for open surgical drainage. Fluoroscopic, ultrasonic, or CT guidance (Fig. 9.17) may be used. The method used depends on the experience and preference of the radiologist and on the size and location of the cavity to be drained. Complications of renal abscess drainage include the exacerbation of urosepsis and hemorrhage.
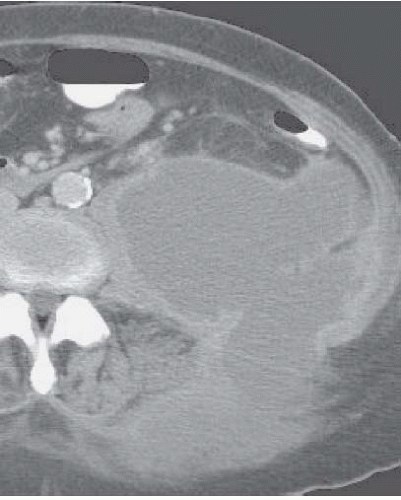 FIGURE 9.15. Left perinephric abscess. An enhanced CT examination reveals a large left posterior perinephric abscess with involvement of the abdominal wall and paraspinal muscles. |
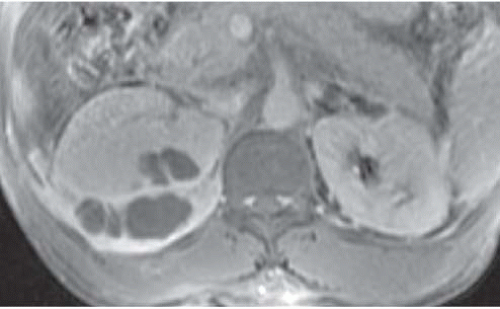 FIGURE 9.16. Perirenal and renal abscesses; T1-weightedgadolinium-enhanced MRI. The pus is hypointense, and the inflamed rims of tissue enhance intensely. |
Pyonephrosis
The term pyonephrosis refers to a pus-filled obstructed renal collecting system. The clinical seriousness of the condition varies, but when severe, it constitutes a true urologic emergency; if untreated, it may lead to sepsis and death. Most patients with pyonephrosis have clinical evidence of urinary tract infection. Calculi are the cause of the associated urinary tract obstruction in a majority of cases; metastatic disease, postoperative ureteral strictures, and processes such as retroperitoneal fibrosis account for the remainder.
Plain abdominal radiographs demonstrate obvious urinary tract calculi in approximately one half of the patients. Sonography may differentiate pyonephrosis from sterile hydronephrosis by demonstrating echoes in the collecting system lumen, which may either be diffuse or appear as dependent layers of debris, or by shadowing echogenic gas bubbles within the pus in case of emphysematous infection (Fig. 9.18).
On CT, a grossly dilated collecting system that contains a urinedebris level or an air-fluid level suggests the diagnosis (Fig. 9.19). CT usually shows evidence of hydronephrosis (Fig. 9.20) and generally demonstrates the cause and level of the associated obstruction. Although rare, layering of contrast medium above purulent material in the collecting system allows a specific diagnosis of pyonephrosis. Percutaneous aspiration of infected urine using radiologic guidance is the definitive diagnostic study in suspected pyonephrosis. Treatment requires drainage by nephrostomy or ureteral stenting along with antibiotics. If percutaneous nephrostomy is performed, care should be taken not to distend the collecting system by injecting contrast because patients may develop serious complications from the procedure including frank sepsis and septic shock.
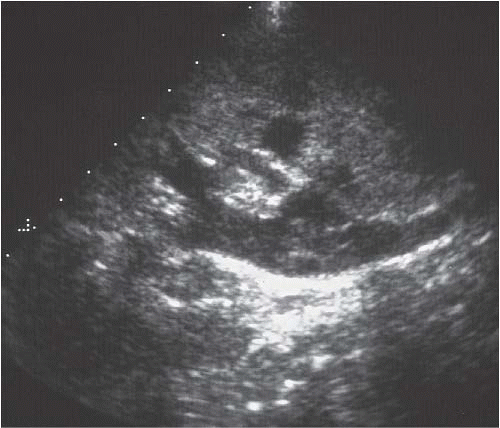 FIGURE 9.18. Pyonephrosis. Longitudinal ultrasound image shows a dilated renal collecting system with multiple, low-level echoes within the collecting system.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|