Renal Inflammatory Disease
Inflammatory disease involving the kidney can be divided into two broad groups: (1) glomerulonephritis, which involves an immunologic injury of the glomerulus, and (2) interstitial nephritis, which is the effect of an infectious or toxic agent on the renal parenchyma. Radiologic studies play a limited role in the diagnosis and management of glomerulonephritis (see Chapter 8). Interstitial nephritis is divided into two major subgroups: (1) noninfectious interstitial nephritis, which is usually caused by the action of toxic agents on the kidney, and (2) infectious interstitial nephritis, which is the result of the action of a pathogen. In most cases, infectious interstitial nephritis is caused by a bacterial organism and is called acute pyelonephritis. This chapter will concentrate on renal infections.
BACTERIAL INFECTIONS
Pathophysiology
Urinary infections are extremely common. Most are limited to the bladder, but in a minority of cases, bacterial infection involves the renal parenchyma, causing acute pyelonephritis. In patients younger than 50 years of age, bacterial infections of the kidney are much more common in women than in men, presumably because of the relatively short length of the female urethra. Beyond this age, however, the incidence of urinary tract infection in men increases, often as a result of urinary stasis caused by bladder outlet obstruction from benign prostatic hypertrophy. Bacteria in the urine are not completely evacuated during micturition and remain in the bladder where they cause cystitis.
Bacteria most commonly reach the kidney through the ureter as a result of ascending infection from the lower urinary tract. In children, this usually occurs as a result of vesicoureteral reflux; in adults, however, frank reflux is uncommon, and bacteria are thought to ascend to the kidney through the ureter against the antegrade flow of urine. Much less commonly, bacterial infections are spread to the kidney hematogenously. Gram-negative enteric pathogens, including Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, and Klebsiella spp., are responsible for the vast majority of bacterial renal infections.
Patients often present with fever, flank or abdominal pain, chills, and other systemic symptoms such as nausea, vomiting, and malaise. These symptoms occur in patients with upper urinary tract infection and help to differentiate them from those infections involving only the lower urinary tract. In most cases, symptoms, clinical signs, and laboratory studies permit a confident diagnosis, and imaging is not needed. There is usually a prompt response to appropriate antibiotic therapy.
Acute bacterial pyelonephritis involves infiltration of the renal interstitium with neutrophils. The kidney becomes edematous; and small microabscesses may form. The process may be unilateral or bilateral and focal or widespread; its distribution is usually patchy, so that involved areas are interspersed with zones of unaffected renal tissue. Although most cases of renal infection produce changes in the renal parenchyma, occasionally just the walls of the ureter and collecting system are involved (pyelitis or pyeloureteritis), producing edema, inflammation, and radiologically visible thickening. Uncommonly, there may be coalescence of the small microabscesses with consequent tissue liquefaction, so that an acute renal abscess appears. During abscess formation, fibroblasts migrate into the area of inflammation to build a wall between the normal parenchyma and the necrotic tissue. This “walling-off” process is characteristic of chronic abscesses, and the remainder of the renal parenchyma returns to normal. If the abscess breaks through the renal capsule, a perinephric abscess is formed. In patients with pyelonephritis and ureteral obstruction sufficiently severe that the affected kidney is oliguric or anuric, the collecting system becomes filled with pus (obstructive pyonephrosis). If pyelosinus extravasation occurs, a perinephric abscess may be formed by this mechanism as well.
Imaging Approach to Renal Inflammatory Disease
In most cases of urinary tract infection, imaging is not indicated. But if there is incomplete or slow response to therapy, multiple repeated infections, immune compromise (especially due to diabetes mellitus), or history of any condition, which may have altered
urinary tract anatomy (surgery, vesicoureteral reflux, stones), renal imaging studies are often required for diagnosis and management. The rationale for performing an imaging study is not only to diagnose acute pyelonephritis but also to look for an underlying anatomic abnormality that may have predisposed the patient to the infection, to search for a calculus or an obstruction that may prevent a rapid therapeutic response, or to diagnose a complication of the infection such as a renal or perinephric abscess. If the investigation is confined to those patients whose fever does not lyse within 72 hours of appropriate antibiotic therapy, the proportion of patients with findings that have immediate clinical significance increases significantly.
urinary tract anatomy (surgery, vesicoureteral reflux, stones), renal imaging studies are often required for diagnosis and management. The rationale for performing an imaging study is not only to diagnose acute pyelonephritis but also to look for an underlying anatomic abnormality that may have predisposed the patient to the infection, to search for a calculus or an obstruction that may prevent a rapid therapeutic response, or to diagnose a complication of the infection such as a renal or perinephric abscess. If the investigation is confined to those patients whose fever does not lyse within 72 hours of appropriate antibiotic therapy, the proportion of patients with findings that have immediate clinical significance increases significantly.
Contrast-enhanced computed tomography (CT) or CT urography (CTU) is the imaging study of choice for the diagnosis of acute pyelonephritis or to look for a potential complication of the infection such as a renal or perinephric abscess or emphysematous pyelonephritis.
Conventional gray scale ultrasound detects most instances of abscess, obstruction, and stones in infected patients and sometimes can suggest pyonephrosis by detecting small particles in infected urine but may fail to detect these abnormalities if they are minor or if the patient is difficult to examine. Sonography is less sensitive than CT in revealing foci of inflamed renal parenchyma, although its accuracy can be improved with ultrasound contrast agents. The modality is safe and relatively inexpensive and can diagnose most of the abnormalities that require specific treatment and thus remains a commonly used technique for patients with renal infection.
Other imaging studies are of value in selected patients. Magnetic resonance imaging (MRI) may demonstrate infected regions of the renal parenchyma to enhance abnormally and to have restricted diffusion and can demonstrate obstruction and abscesses but often fails to reveal small stones; the technique should be reserved for patients who cannot undergo contrast-enhanced CT examinations. Retrograde pyelography may serve as both diagnostic and planning maneuvers in patients with obstructive pyonephrosis who are to be treated with ureteral stents or percutaneous nephrostomy tubes, respectively. Voiding cystourethrography is used to demonstrate vesicoureteral reflux but is performed only rarely in adults.
In children with multiple urinary tract infections, the classic imaging approach involves searching for reflux and other bladder and urethral abnormalities with voiding cystourethrography. Demonstration of reflux often leads to a search for renal infection and scars with renal scintigraphy (usually with technetium-99m dimercaptosuccinic acid [99mTc-DMSA]) and/or renal gray scale and Doppler ultrasound. There is a growing tendency to use a “top-down” approach to imaging, in which renal abnormalities are sought with ultrasound or scintigraphy, and efforts to diagnose reflux are reserved for patients with renal findings that suggest it. Professional society guidelines are not unanimous on this issue.
Acute Pyelonephritis
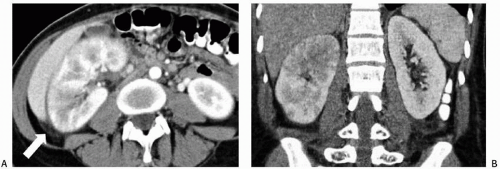
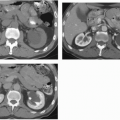
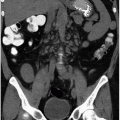
The imaging examination providing the most complete information regarding the nature and extent of the inflammatory process is contrast-enhanced CT. Unenhanced scans may be normal or show only renal enlargement (Fig. 7.1); there may be perinephric stranding from inflammation or edema (Fig. 7.2), and Gerota’s fascia may be thickened. After intravenous contrast administration, areas of decreased contrast enhancement appear (Figs. 7.2 and 7.3). These regions may be inhomogeneous or striated and their distribution is variable. They may be solitary or multiple and unilateral or bilateral and may involve little or most of the renal parenchyma. The distribution is typically patchy. Diffuse homogeneous involvement of the renal parenchyma is rare and is likely to appear only when there is ureteral obstruction. If delayed scans are obtained, the abnormal areas may reveal a dense nephrogram, which persists
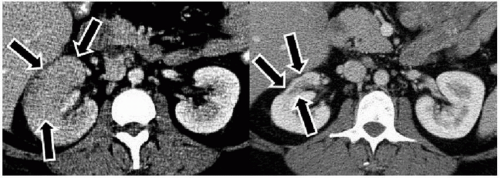
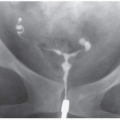
after the normal parenchyma has lost its enhancement (Fig. 7.4). Differential diagnosis of regions of diminished or inhomogeneous enhancement may include acute ischemia or contusion; if there is focal swelling, a focus of acute pyelonephritis may resemble an infiltrating tumor (tumefactive pyelonephritis). Clinical data often permit a specific diagnosis. Acute ischemia may be indicated by the peripheral rim sign or visible vascular abnormalities and produce fewer changes than pyelonephritis in the perinephric fat. The pyelitis which sometimes accompanies renal infection may be reflected by thickening of the walls of the collecting system (Fig. 7.5).
after the normal parenchyma has lost its enhancement (Fig. 7.4). Differential diagnosis of regions of diminished or inhomogeneous enhancement may include acute ischemia or contusion; if there is focal swelling, a focus of acute pyelonephritis may resemble an infiltrating tumor (tumefactive pyelonephritis). Clinical data often permit a specific diagnosis. Acute ischemia may be indicated by the peripheral rim sign or visible vascular abnormalities and produce fewer changes than pyelonephritis in the perinephric fat. The pyelitis which sometimes accompanies renal infection may be reflected by thickening of the walls of the collecting system (Fig. 7.5).
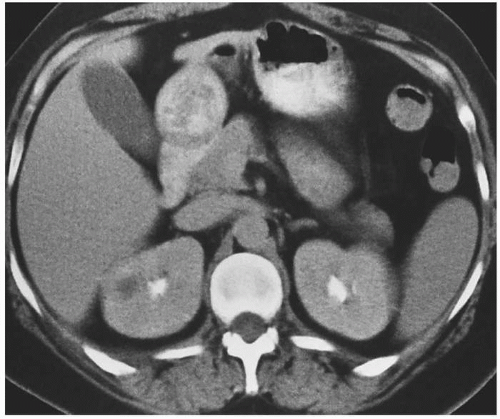
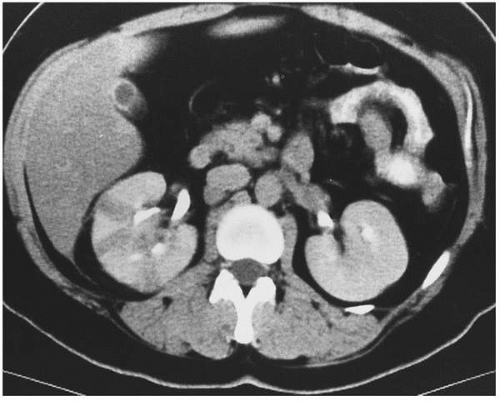 FIGURE 7.3. Acute pyelonephritis. Relatively narrow striated areas of decreased enhancement are seen in the right kidney. |
The appearance of pyelonephritis on CT may be modified by antibiotic therapy. Partially treated patients may demonstrate a rounded or ovoid area of decreased enhancement with poorly defined margins (Fig. 7.6). Long-term follow-up studies in patients with mild disease may show a return to normal renal morphology and enhancement (Fig. 7.7). With more severe disease, focal atrophy may appear at the sites of previous infection (Fig. 7.8) and there may be focal calyceal clubbing, suggestive of papillary necrosis. The etiology of these morphologic changes has been postulated to involve ischemic insult to the kidney as a result of the inflammatory process. Ultrasound in patients with acute uncomplicated pyelonephritis may be normal or show diffuse or focal renal enlargement with regions of increased or decreased echogenicity of the renal parenchyma (Fig. 7.9). Perinephric inflammation may produce a thin layer of extracapsular fluid adjacent to the infected portions (Fig. 7.10). The normal corticomedullary differentiation may be lost. Doppler ultrasound may reveal focal regions of abnormal perfusion (Fig. 7.9); although there is hyperemia of the affected regions, the normal renal parenchymal flow is so high that the infected regions may have less flow than surrounding normal tissue, perhaps due to elevated interstitial pressure from edema.
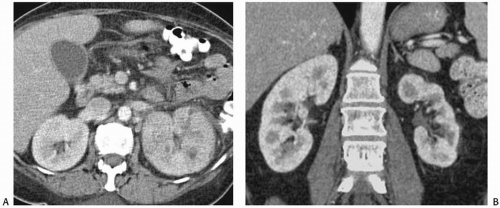
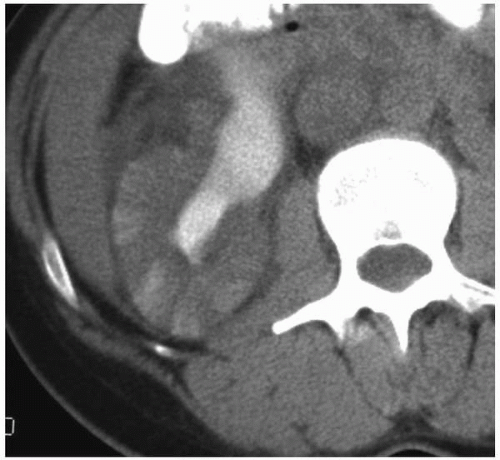 FIGURE 7.4. Acute pyelonephritis; delayed CT. The abnormal regions are revealed by wedge-shaped regions of retained parenchymal contrast. |
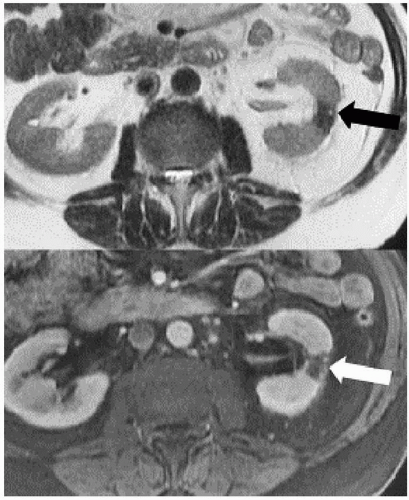
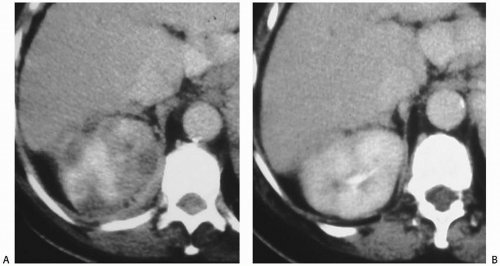 FIGURE 7.7. Acute pyelonephritis. A: Before treatment, the kidney is severely affected, with regions of markedly reduced enhancement. B: After treatment, morphology and enhancement are normal. |
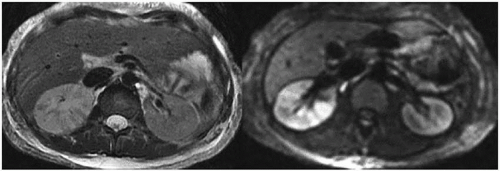
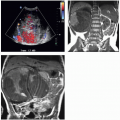
The MRI findings are morphologically similar to those seen on CT and reflect swelling, altered perfusion, and focal areas of diminished enhancement (Fig. 7.11). Diffusion-weighted images reveal restricted diffusion in the affected portions (Fig. 7.11) and may be more sensitive than either T2-weighted or contrastenhanced T1-weighted images (Fig. 7.12) for detecting regions of pyelonephritis.
Radionuclide imaging in patients with acute pyelonephritis has been reported using renal cortical imaging agents, such as 99mTc-DMSA, and agents that image inflammation, such as gallium (67Ga) citrate. Renal cortical imaging studies may show an inhomogeneous distribution of the radionuclide within the affected kidney or polar defects with asymmetric tracer uptake.
Chronic Pyelonephritis
The term chronic focal atrophic pyelonephritis applies to renal parenchymal scarring in which the cortex and medulla are focally thinned and the underlying calyces are blunted. This appearance may have a number of causes, including vesicoureteral reflux, calyceal stones, or severe focal pyelonephritis (Figs. 7.8B and 7.13). Once the scars have appeared, the gross pathologic and radiologic appearance of the affected kidney does not allow determination of the cause; microscopy reveals focal fibrosis in all cases. If the etiology is known to be vesicoureteral reflux, the term reflux nephropathy is used. It is thought that as urine refluxes during bladder contractions, the hydrostatic pressure in the collecting system rises transiently, and the urine, which is often infected, flows retrograde into the collecting tubules of the kidney (pyelotubular backflow or reflux). Such intrarenal reflux occurs in a patchy distribution, possibly because of local differences in the papillary openings of the collecting ducts of the renal medullae, which in turn is probably responsible for the focal nature of the resultant scarring. Within the scarred regions, dense fibrous tissue replaces normal nephrons (Fig. 7.14).
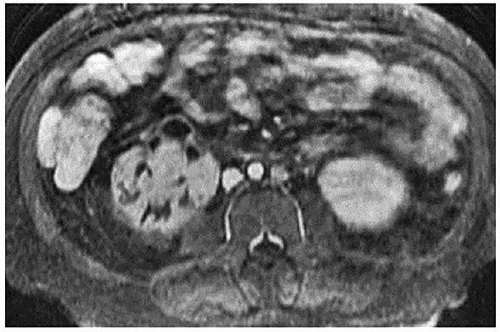 FIGURE 7.12. Acute pyelonephritis. Contrast-enhanced T1-weighted image with fat saturation reveals regions of poor or absent perfusion in the right kidney. |
Calyceal stones, possibly by causing elevated calyceal pressure due to infundibular obstruction or by acting as foci of infection, frequently cause calyceal clubbing and scars that are indistinguishable from reflux nephropathy (Fig. 7.15). When chronic pyelonephritic scarring is found in middle-aged or elderly patients, stones are most likely to be the cause; in children, reflux has usually produced the
scars. Focal infarction may produce similar scars; however, calyceal blunting is less likely to be found in this condition. Trauma, including surgery, and radiation therapy, which includes portions of the kidney (Fig. 7.16), may also produce scars.
scars. Focal infarction may produce similar scars; however, calyceal blunting is less likely to be found in this condition. Trauma, including surgery, and radiation therapy, which includes portions of the kidney (Fig. 7.16), may also produce scars.
The radiologic findings of chronic pyelonephritis on CT, CTU, and MRI include the demonstration of one or more parenchymal scars (Fig. 7.17) overlying a deformed calyx. If parenchymal loss has been severe, focal areas of compensatory hypertrophy may be seen adjacent to the areas of cortical scarring. These may mimic solid parenchymal tumors but can be distinguished from tumors by observing that when contrast is administered, they enhance to the same degree as adjacent normal parenchyma.
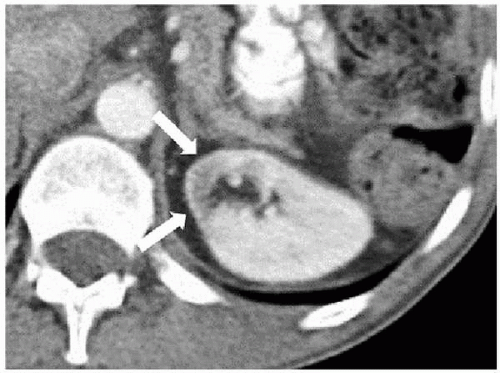 FIGURE 7.16. Radiation-induced scar. The medial aspect of the kidney (arrows) was included in a radiation therapy field several years earlier and is now atrophic and has diminished opacification. |
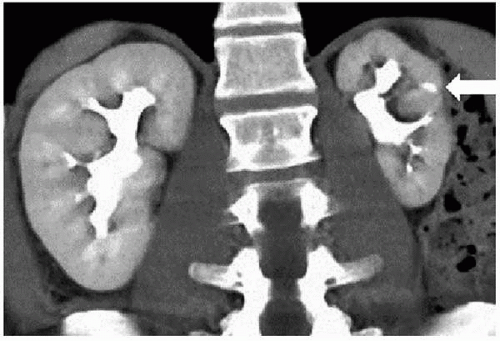 FIGURE 7.17. Chronic focal atrophic pyelonephritis due to childhood reflux. The left kidney is small and demonstrates focal parenchymal loss (arrow) with a blunted calyx. |
Ultrasound changes in patients with chronic atrophic pyelonephritis include focal loss of parenchyma, which can be appreciated on longitudinal or cross-sectional images. Increased echogenicity in the area of the scar may also be demonstrated. The central renal sinus echoes may extend to the periphery of the kidney in the area of abnormality. In contrast to other causes of generalized increased cortical echogenicity, the process appears focal.
Acute Renal Abscess
Renal abscesses form as a result of the coalescence of microabscesses that are often present in acute pyelonephritis. The predominant organisms responsible for abscesses are gram-negative enteric species. Patients with diabetes mellitus, drug abuse, vesicoureteral reflux, or renal calculus disease are most susceptible to abscess formation. Acute abscesses may be solitary or may form simultaneously in multiple locations in the kidney. Multiple lesions are less common and suggest hematogenous dissemination.
The signs and symptoms of an acute renal abscess are difficult to distinguish from those of pyelonephritis, and the diseases often coexist. Fever, leukocytosis, pyuria, and flank pain are common in both. There is frequently a history of prior antibiotic therapy with recrudescence of symptoms on cessation of treatment. Renal abscesses appear as a continuum of conditions. Acute pyelonephritis may progress to a cluster of small abscesses, which may or may not coalesce, so that there may be a single fluid-filled cavity, a multiloculated cavity, or a phlegmonous region in which a network of solid tissue planes separate multiple small abscesses. When fibroblasts migrate into the area of an acute renal abscess and form a barrier between the abscess and the remainder of the kidney, a chronic renal abscess is formed. This may result in a transition zone of inflammatory tissue between the liquid center of the abscess and the normal renal parenchyma.
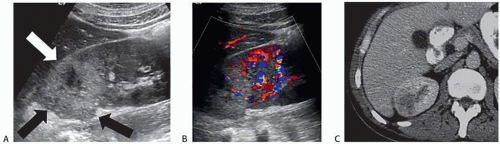
On sonography, abscesses appear as relatively sonolucent lesions with differing amounts of solid tissue echoes; the pus may contain low-amplitude echoes (Fig. 7.18) and there may be gas bubbles. There is usually enhanced through-transmission of the ultrasound beam. Doppler studies show no flow in the necrotic pus-filled regions; the amount of flow in the wall is variable.
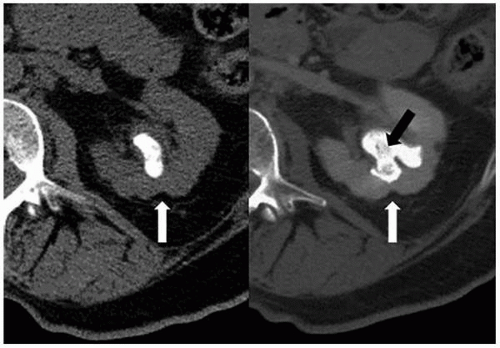
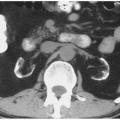
CT is the imaging study of choice for the diagnosis of an acute renal abscess. The lesion is a low-attenuation (10 to 20 HU) rounded or ovoid mass that does not enhance with contrast administration (Fig. 7.19). As a result of the surrounding inflammatory process, the borders of the mass are usually indistinct; the degree
of enhancement of the abscess wall is variable. The presence of gas within a pocket of fluid is virtually pathognomonic of an abscess. There is usually thickening of Gerota fascia, and increased density may be found in the adjacent perinephric and mesenteric fat. The abscess may or may not extend into the perinephric space. MR findings reflect those seen on CT. The center of the abscess has the characteristics of fluid, and the periphery of the lesion, which is of varying thickness, enhances to varying degrees (Figs. 7.19 and 7.20). Radionuclide scanning with technetium-labeled DMSA is occasionally used but has largely been replaced by CT.
of enhancement of the abscess wall is variable. The presence of gas within a pocket of fluid is virtually pathognomonic of an abscess. There is usually thickening of Gerota fascia, and increased density may be found in the adjacent perinephric and mesenteric fat. The abscess may or may not extend into the perinephric space. MR findings reflect those seen on CT. The center of the abscess has the characteristics of fluid, and the periphery of the lesion, which is of varying thickness, enhances to varying degrees (Figs. 7.19 and 7.20). Radionuclide scanning with technetium-labeled DMSA is occasionally used but has largely been replaced by CT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree