Fig. 1
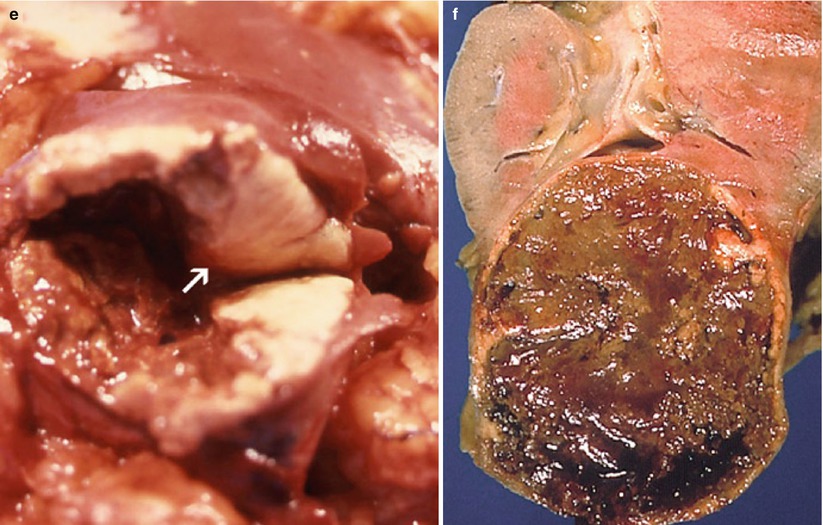
(a–f) Cystic renal neoplasms. Gross anatomy of the most common subtypes. (a) Multilocular cystic nephroma (consistent with Bosniak category III). (b) Multilocular cystic clear cell carcinoma (consistent with Bosniak category III). (c) Cystic RCC with multilocular cystic pattern (consistent with Bosniak category IV). Note the presence of yellow-colored solid elements ( arrows) composed of carcinomatous tissue. (d) Cystic RCC with unilocular cystic pattern (consistent with Bosniak category IV). Note the presence of a thick irregular wall with nodularity (arrows). (e) Pseudocystic RCC due to massive necrosis (consistent with Bosniak category IV). Note the presence of a mural solid nodule composed of tumor tissue (arrow). (f) Pseudocystic RCC due to massive necrosis (consistent with Bosniak category III). Note the absence of solid tumor tissue elements. The lesion is surrounded by a thick wall with slight irregularity
Among benign renal tumors, those presenting with a cystic pattern are multilocular cystic nephromas (MCN) and mixed epithelial and stromal tumors (MEST). Both tumors are deemed to represent opposite ends of the spectrum of the same entity. A unifying term “renal epithelial and stromal tumors” (REST) has been recently proposed to designate this group of neoplasms (Turbiner et al. 2007; Montironi et al. 2008).
MCN, also called cystic nephroma or multilocular cyst, is characterized by a multiseptated fluid-filled mass surrounded by a thick fibrous capsule. The fluid content of loculi consists of pale yellow serous fluid or, less commonly, myxomatous fluid. The cystic component is lined with a flattened unistratified cuboidal or hobnail cell epithelium and septated by fibrous septa with ovarian-type stroma. On gross inspection, solid or nodular elements are not found. MCN occurs most commonly in women over the age of 30 years.
MEST is characterized by an epithelial-lined tubular or cystic component combined with an abundant stroma of variable cellularity and growth patterns. The stromal proliferation is responsible for the presence of a solid component in a high proportion of cases. MEST also occurs with a strong female predominance typically in perimenopausal women (Lane et al. 2008; Montironi et al. 2008).
Both entities are usually large and have the propensity to extend within the renal sinus and herniate into the renal pelvis unlike any other renal tumor (Alanen et al. 1987; Park et al. 2005). Such pattern has been reported to be highly suggestive of REST.
2.2.3 Malignant Cystic Neoplasms (Fig. 1)
Approximately 4–15 % of RCCs show a cystic pattern. Cystic RCCs include one specific entity introduced in the 2004 WHO classification – the multilocular cystic renal cell carcinoma – and other variants of RCCs that can exhibit cystic changes due to various mechanisms (Hartman et al. 1986, 1987; Hartman 1990; Freire and Remer 2009; Lopez-Beltran et al. 2006). A recently introduced new entity – the tubulocystic carcinoma – is a rare malignant cystic neoplasm not yet included in the WHO classification of renal tumors.
Multilocular Cystic RCC of Low Malignant Potential
This uncommon (1–4 % of RCCs) low-grade variant of clear cell carcinoma has an excellent prognosis. It is usually incidentally discovered in adults (mean age 51 years). Macroscopically, it does not differ from MCN. The cysts are lined by a single layer of epithelial cells with clear to pale cytoplasm. A few small papillae can also be found but without any expansive solid nodules.
Clear Cell or Papillary RCCs with Cystic Changes
RCC of the clear cell type is the most common RCC accounting for about 75 % of all RCCs. It is the most common subtype of RCC that can exhibit a cystic pattern either with a multilocular or unilocular presentation. Papillary RCC is a less aggressive variant characterized by a tubulopapillary growth pattern with two cellular subtypes (types I and II) as described in the current WHO classification. The reported incidence of papillary RCC is 10 %. Multicentric and bilateral papillary RCCs are not uncommon. Internal changes such as hemorrhage, necrosis, and cystic degeneration are frequently observed in large tumors.
RCCs (clear cell) with multilocular cystic pattern: The cystic component results from an intrinsic multilocular growth of the tumor. Macroscopically, the tumor is composed of thick-walled septated cysts that are filled with serous or hemorrhagic fluid. Grossly recognizable solid component such as irregular thick portions of septa or wall and soft tissue nodules can be found. Histopathologic analysis shows carcinomatous proliferation of malignant epithelial cells arranged in variable patterns.
RCCs (clear cell or papillary) with unilocular cystic pattern: Three mechanisms by which RCC exhibits a unilocular cystic pattern have been described (Silverman and Kilhenny 1969; Weitzner 1971; Varma et al. 1974; Sufrin et al. 1975; Levy et al. 1999): an intrinsic unilocular cystic growth of the tumor, a massive tumor necrosis, and carcinomas arising from the epithelium of a preexisting renal cyst.
Tubulocystic Carcinoma
Tubulocystic carcinoma has also been described under the term low-grade collecting duct carcinoma. This rare subtype of RCC is a new tumor entity not recognized in the WHO 2004 classification. It occurs in adults (mean age, 54 years) with a strong male predominance (7:1). Grossly, the tumor exhibits a spongy or “bubble wrap” appearance reflecting the microscopic presence of variably sized cysts and dilated tubules separated by delicate septa (Sibony and Vieillefond 2008; Amin et al. 2009).
3 Imaging Techniques and Key Interpretation Criteria
3.1 Conventional Ultrasound
Because of the widespread use of US in routine examination of the abdomen (that includes kidney evaluation), the detection rate of renal masses in asymptomatic patients has dramatically increased. Fortunately, US provides definitive diagnostic information in most of the simple renal cysts that are incidentally screened.
Recent technical improvements of grayscale imaging have increased US performance in the detection and characterization of cystic renal masses.
Nonlinear US technique (tissue harmonic imaging (THI), differential THI, pulse inversion imaging) is now a widely implemented modality that provides a better contrast resolution and less artifacts on grayscale image enabling to eliminate “dirty echoes.” Accuracy for classifying cystic masses has been reported to increase from 64 % to 84 % by using pulse inversion imaging (Schmidt et al. 2003). In addition to its better capabilities in characterizing cystic renal masses, this modality may help depict and characterize very small cysts.
Typically, the simple cyst exhibits anechoic content, enhanced through transmission of the US beam resulting in posterior acoustic enhancement and smooth and sharply defined margins without perceptible wall (Fig. 2).
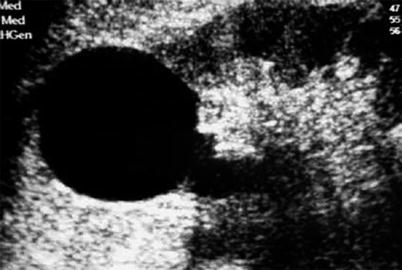

Fig. 2
Ultrasonography (US) of a simple cyst. US shows an anechoic renal mass with posterior acoustic enhancement and homogeneous pattern without perceptible wall
Cystic renal masses that do not fulfill the criteria for a simple cyst remain indeterminate at US and require further contrast-enhanced imaging workup. However, there is some US criteria that can strongly suggest a cystic or pseudocystic renal neoplasm including (Helenon et al. 2001) (Figs. 3 and 4a) a thick irregular peripheral wall or multiple thick septa, the presence of echoic mural nodules, and the demonstration of vascular flow signal within the solid component of the lesion at color Doppler US.
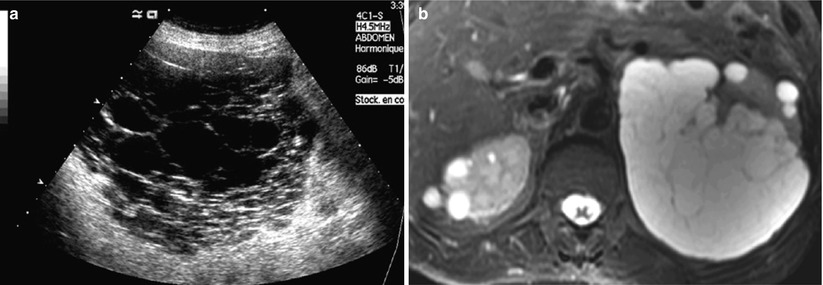
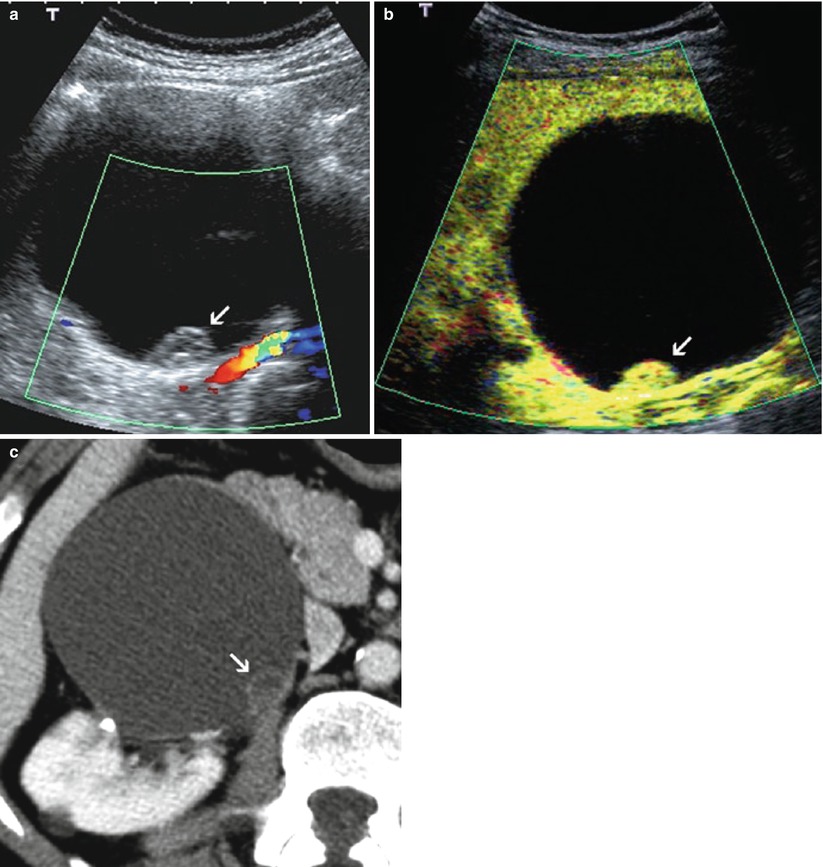

Fig. 3
(a, b) US of a multilocular cystic renal mass. (a) US shows a septated multilocular cystic mass. (b) MRI (T2-weighted axial image) demonstrates the presence of multiple smooth septa with moderate enhancement (refer to Fig. 17e)

Fig. 4
(a–c) Contrast-enhanced US of a cystic RCC. (a, b) Unenhanced color Doppler US (a) and postcontrast US (b) show a cystic renal mass with enhancing mural nodule (arrow) consistent with a cystic RCC. (c) Computed tomography (CT) confirmation of a complex cystic mass of Bosniak category IV. Note the slight enhancement of the solid component (arrow) after iodine contrast administration. The enhancing nodule is seen much better on contrast-enhanced US because of its higher sensitivity to microbubble signal enhancement
US may also provide additional diagnostic information over CT in selected cases among complex cystic renal masses that remain equivocal at CT (Hélénon et al. 2001). On the other hand, because of the higher sensitivity of US in detecting cyst septations, some cystic masses can appear more complex at US than at CT. Such discrepancy, however, should not be considered in the differentiation between surgical and nonsurgical complex cystic masses, although it can lead to the upgradation of a nonsurgical minimally complex cyst into a category that should be followed up with CT or at best first reevaluated with MRI (refer to Sect. 5.4).
3.2 Contrast-Enhanced Ultrasound
Intravenous ultrasonography contrast agents (USCAs) are useful in characterizing complex cystic masses since they improve the sonographic depiction of solid tissue vascularity. Real-time contrast-enhanced US at low MI using second-generation USCAs provides better resolution and longer persistence compared to the first introduced dedicated US sequences and contrast agents (Correas et al. 2006). Contrast enhancement of the wall or septa, usually assessed by CT or MRI, is the key criteria in determining whether the lesion needs to be removed surgically or followed. The high sensitivity of contrast-enhanced US in detecting microbubble signal from the vascularized wall, septa, or solid nodules (Fig. 4) improves lesion classification and may change therapeutic options (Correas et al. 2006; Ascenti et al. 2007; Quaia et al. 2008; Cazals et al. 2011). USCAs can therefore play a critical role in the characterization of complex cystic masses. However, contrast-enhanced US may be too sensitive as it can detect only a few microbubbles traveling in thin septations with a superior time and spatial resolution compared to any other imaging modalities. Such a finding may lead to overclassify certain cases of benign cysts.
The recently reported performance of the technique in evaluating complex cystic masses suggests that contrast-enhanced US is appropriate in the Bosniak classification system. In a recent study, Quaia et al. (2008) reported a better overall accuracy of CEUS compared to CT in the diagnosis of malignancy in complex cystic renal masses. CEUS can be currently proposed as an alternative to CT in the follow-up of complex renal cysts and in patients with serious contraindication to contrast-enhanced CT or MRI (Quaia et al. 2008). Moreover, it can be helpful in the follow-up of indeterminate cystic masses in order to reduce the radiation dose (Ascenti et al. 2007).
Some limitations of the technique including back shadowing from wall calcification, bowel gas interposition, and US beam attenuation in cases of deep lesion location can obscure contrast enhancement after microbubble injection. On the other hand, another pitfall can result from strong enhancement of the surrounding vessels or normal parenchyma that should not be confused with thick wall enhancement.
3.3 Computed Tomography
CT remains the gold standard in detecting and characterizing cystic renal masses (Warren and McFarlane 2004; Hartman et al. 2004; Israel and Bosniak 2005b; Helenon et al. 2008). The Bosniak classification system is based on specific CT criteria that rely on the cystic lesion enhancement properties and morphologic features. This classification scheme will be discussed in more detail later.
Proper CT technique is needed for accurate categorization of cystic masses. Appropriate dedicated renal CT technique using multidetector row CT includes both unenhanced and contrast-enhanced scans with adequate bolus injection (at least 100 mL of a 300–350 mg iodinated contrast medium) delivered at a rate of 2–4 mL/s; multiphasic acquisition including at least unenhanced, postcontrast arterial (or corticomedullary) phase (approximately 30–40 s after the start of injection) and nephrographic phase (80–100 s) with additional delayed excretory phase in selected cases (e.g., when contrast enhancement remains doubtful or in case of a suspected calyceal diverticulum); and thin section thickness (≤3 mm thick with 0.5–1.5 overlap).
The most relevant postcontrast phase in terms of diagnostic performance is the parenchymal nephrographic phase that can be sufficient in combination with unenhanced phase, in the assessment of most of renal cystic masses (Kim et al. 2013).
Regarding interpretation technique and limitations, one should keep in mind that attenuation variation can result from volume averaging, motion, and beam-hardening artifacts. The latter can be responsible for pseudoenhancement that can reach up to +28 HU as assessed in a phantom model (Maki et al. 1999; Bae et al. 2000; Coulam et al. 2000; Heneghan et al. 2002; Gokan et al. 2002; Birnbaum et al. 2002; Abdulla et al. 2002) and +20 HU in vivo (Fig. 5). Reported in vivo predictors of pseudoenhancement include the renal cyst size and endophytic growth pattern, the background nephrographic phase parenchymal attenuation, and the number of scanner detector rows (pseudoenhancement increases with larger numbers of detectors) (Tappouni et al. 2012; Sai et al. 2013; Patel et al. 2014).
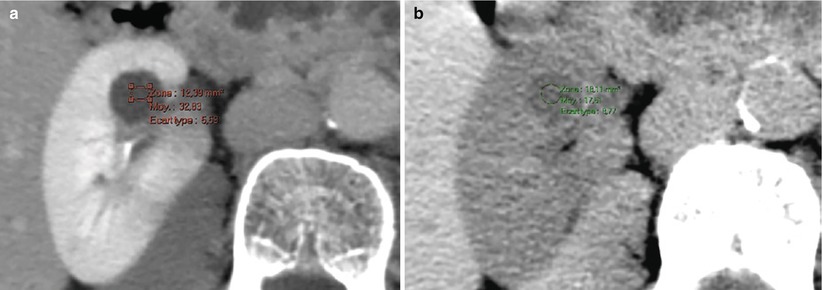

Fig. 5
(a, b) Pseudoenhancement of a simple renal cyst at CT. (a) Contrast-enhanced CT (nephrographic phase) shows an increase in attenuation numbers of 15 HU compared to unenhanced evaluation (b)
As recently reported by Jung et al. in a phantom study, virtual monochromatic image obtained with dual-energy CT (DECT) has the potential to decrease renal cyst pseudoenhancement (Jung et al. 2012). When available, DECT therefore can be useful to better characterize small renal cysts.
Imperfect placement of the ROI and evaluation of CT numbers on inadequate image acquisition, especially the early corticomedullary phase, can provide misleading information. Quantitative evaluation of attenuation variations between unenhanced and postcontrast images can also provide misleading information on masses that exhibit subtle enhancement of a limited internal portion not included within the ROI.
Some recommendations and general principles can therefore be made to avoid such pitfalls: Attenuation values in very small lesions (i.e., ≤5–10 mm) should be considered unreliable to provide accurate characterization; accurate evaluation of enhancement needs constant setting of exposure (kilovoltage and milliamperage), field of view, position in gantry, and section thickness; measurements of ROI should be obtained in comparable portions of the lesion to assess enhancement; visual appreciation of attenuation variations using narrow window settings is also needed to identify enhancement within limited portions of the mass; the postcontrast images on which enhancement should be appreciated are those obtained at a nephrographic phase although all other contrast-enhanced images (depending on scanning protocol) need to be at least visually analyzed.
Analysis of CT features that lead to recognize and categorize cystic masses relies on internal attenuation values, enhancement properties, and morphologic features. Basic interpretation criteria are defined as follows:
“Water” attenuation values are defined between −10 and +20 HU (practically, −5 to +15 HU).
A lack of enhancement usually indicating the absence of vascularity (except for some rare poorly vascularized solid tumors) if defined by attenuation variation less than +10 HU after the administration of contrast material.
A significant enhancement indicating vascularity and, therefore, the solid nature of a mass is defined by a change in attenuation of more than +20 HU after injection.
Postcontrast attenuation variation assessed between +10 and +20 HU should be viewed as indeterminate and therefore prevent separate true cystic lesions (with pseudoenhancement) and some solid neoplasms with poor vascularity.
Enhancement characteristics of a known cystic mass are a major factor of evaluation and categorization. Wall and septa are generally appreciated only on postcontrast images because of their enhancement even in lesions categorized in minimally complicated benign cysts. The level of enhancement of soft tissue elements (wall, septa, or nodules) can be subjectively assessed as follows: minimal enhancement that can be perceived (not measurable), significant measurable enhancement usually of grossly thickened wall or septa, or solid elements.
“Thick wall” is a descriptive term that encompasses different degrees of thickening. The wall of a simple cyst is macroscopically smooth and thin (<1 mm) and should remain imperceptible at imaging (US, CT, or MRI). The different degrees of thickening, which are consistent with complex cystic masses, include (Fig. 6) minimal smooth wall thickening also described as “perceptible” (not measurable) or “hairline-thin” (≤1 mm) wall, grossly thickened and uniform wall, slightly irregular thick wall, and marked irregular thick wall with solid elements (nodularity).
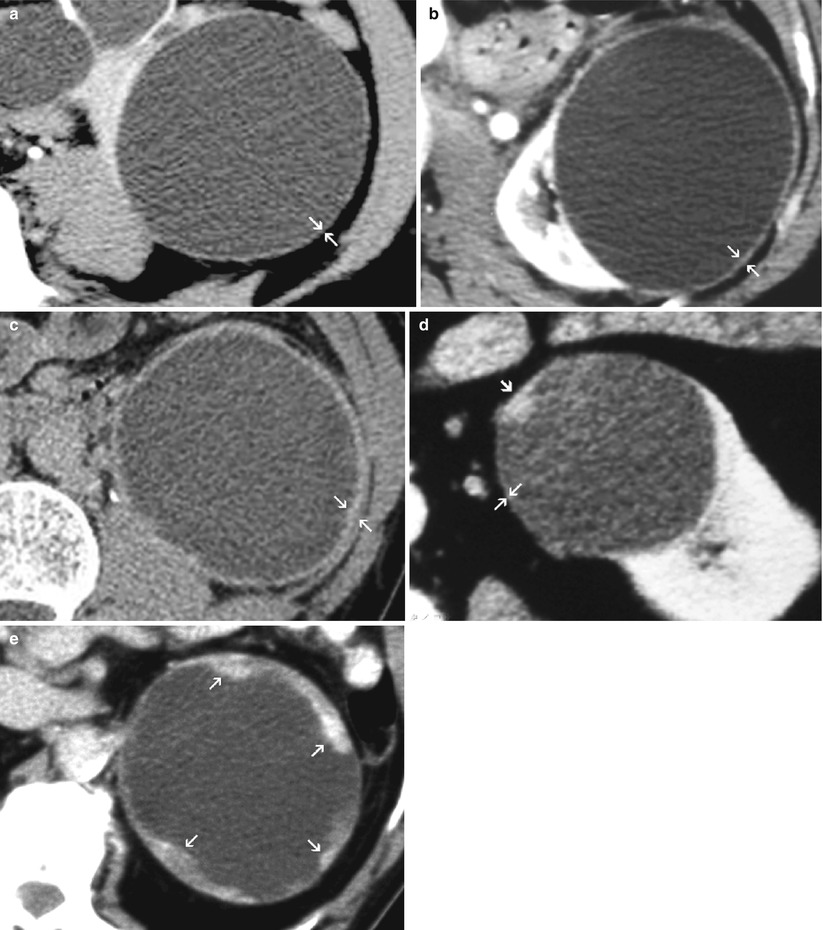
Fig. 6
(a–d) Patterns of wall thickening in complex cystic masses. (a) Minimal “hairline-thin” (≤1 mm) wall thickening (arrows). (b) Grossly thickened uniform and smooth wall (arrows). (c) Grossly thickened wall with slight irregularity (arrows). (d) Mural nodule (arrowhead) (here, arising from a minimally thickened wall) (arrows). (e) Marked irregular thick wall with solid elements (arrows)
Septations also can exhibit different degrees of thickening as mentioned above. The number of septa (few or multiple septa) is another important finding in complex renal cyst evaluation. MR imaging and US are more sensitive than CT in demonstrating septa within a lesion and therefore can cause a lesion to be upgraded (refer to Sect. 5.4).
Calcification is morphologically described as thin and smooth or thick and irregular (Fig. 7). They can involve the cyst wall or septa.
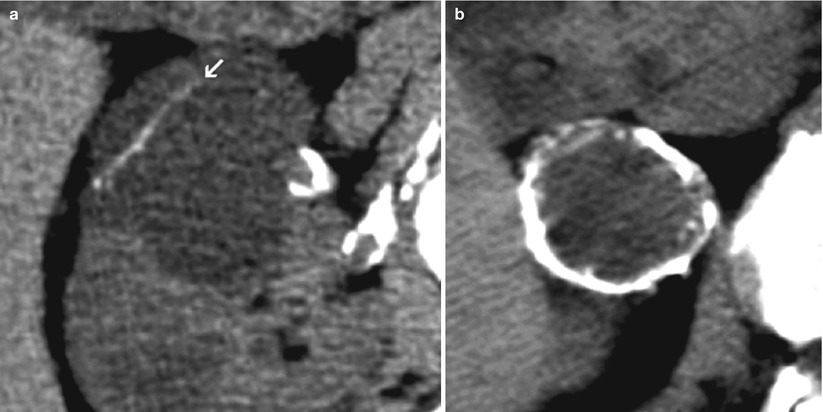
Fig. 7
(a, b) Patterns of cystic calcifications. (a) Thin and smooth calcification of a cyst septum (arrow). (b) Thick irregular calcification of a “cyst” wall (cystic RCC)
3.4 MR Imaging
MRI plays an increasing role in evaluating cystic renal masses. It is now considered at least equivalent to CT in the characterization of cystic lesions (Israel and Bosniak 2004, 2005a, b; Israel et al. 2004; O’Malley et al. 2009). It is commonly used as a substitute in patients with serious contraindication to CT (due to radiation exposure or iodinated contrast agent) and as a “problem-solving” modality especially in masses that remain indeterminate at CT. The increasing use of MRI in abdominal imaging has led to the frequent discovery of incidental cystic renal masses. Therefore, the radiologist should be able to provide accurate and definitive diagnosis in most cases of cystic renal lesions on the basis of MR findings and without the need for further imaging studies.
A high-quality examination is needed to accurately characterize a complex cystic renal mass. The MR examination of a complex cystic renal mass can be performed using a standard protocol dedicated to renal “parenchymal” imaging which consists of fast or turbo spin echo T2-weighted imaging, T1-weighted spoiled gradient echo (GRE) sequence obtained in out of phase, and 3D fat-suppressed T1-weighted GRE before and after gadolinium complex administration (dynamic contrast-enhanced sequence) with a scan delay of about 5 min including serial pre- and postcontrast corticomedullary, nephrographic, and excretory phases. Sequences should be performed using a torso phased-array coil. Whereas T1-weighted GRE sequences are necessarily obtained during breath holding, the T2-weighted acquisition can also be performed with respiratory compensation that minimizes motion artifact while providing better spatial resolution than with breath-held T2-weighted acquisition.
Advantages of MR imaging over CT in terms of imaging performance are its higher contrast resolution and higher sensitivity to detect even subtle contrast enhancement and the capability to better characterize the internal components of complex cystic masses including blood breakdown products and necrotic debris.
Contrast enhancement at MRI is usually appreciated subjectively by comparing side-by-side pre- and postcontrast images, since unquestionable enhancement is frequently apparent. In cases of subtle contrast enhancement, quantitative assessment is needed based on the relative percentage increase of signal intensity after contrast injection. A threshold of 15 % is commonly used to define a significant contrast enhancement as reported in the study of Ho et al. (2002). Image subtraction which is now a widely implemented postprocessing technique can also be useful in assessing subtle postcontrast enhancement and enhancement characteristics of complex cystic lesions that show hyperintense signal intensity on precontrast images.
Diffusion-weighted MRI with ADC measurements provides additional information that may help differentiate complex renal cysts from cystic carcinomas (Sandrasegaran et al. 2010; Inci et al. 2012).
As reported by Gulani et al. (2008), artifactual thickening of the cyst wall can be observed on low-resolution fat-saturated spoiled gradient echo (FS-SPGR) sequences. This apparent increase in the lesion wall thickness is seen in the phase-encoding direction. Image interpretation should therefore take into account the direction of the wall thickening. In case of suspected artifactual thick wall, higher-resolution images (at best 512 × 512) can be acquired to rule out true thickening.
Specific indications of MRI in the diagnosis of complex cystic renal masses will be discussed later (refer to Sect. 5).
4 The Bosniak Classification System
In 1986, Morton Bosniak proposed a classification system of cystic renal masses designed to separate lesions requiring surgery from those that can be left alone or followed (Bosniak 1986). In this classification, cystic lesions were divided into four categories (nonsurgical categories I and II, surgical categories III and IV) by the analysis of specific CT criteria including morphologic and enhancement characteristics.
During the following decade, M. Bosniak first pointed out difficulties in classifying some cystic lesions using the original classification system (Bosniak 1991a) and finally introduced the category IIF (F for follow-up) in 1997 (Bosniak 1997a, b). This category consists of minimally complicated cysts that do not neatly fall into category II, but are not complex enough to fulfill the criteria of category III. In 2003, this follow-up approach of category IIF has been shown by Israel and Bosniak (2003a, b) to be appropriate and safe. It can prevent unnecessary surgery in more than 95 % of complex cysts that belong to this category (Israel and Bosniak 2003a). It enables the identification of cystic neoplasms on the basis of the progression of imaging findings that lead to the upgradation of initial presentation into surgical category III or IV. In another retrospective study, Israel and Bosniak extended the category IIF to cysts with thick irregular or nodular calcifications provided no associated soft tissue or thickened wall or septal enhancement is present (Israel and Bosniak 2003b).
One year later, Israel et al. originated a new important step in the evaluation of cystic renal masses by introducing the role of MRI in the Bosniak classification system (Israel et al. 2004). It has been shown that the classification scheme can be applied to MRI and can benefit from MR information especially in the characterization of category IIF complex cysts. Regarding the reliability of the Bosniak classification system, several studies have been reported between 1997 and the early 2000s (refer to Sect. 4.3). Among these, one major study by Curry et al. (2000) concluded that the Bosniak classification is reliable and requires adequate CT technique.
4.1 Field of Utilization
Utilization of the Bosniak classification system requires, first, previous identification of the cystic nature of a renal mass as defined earlier (refer to Sect. 2.1) with the exception of very small lesions (<5–10 mm) and, second, examination of the cystic lesion if it belongs to the field of application of the Bosniak classification system. The latter has been designed to characterize renal cystic lesions including renal cysts and cystic neoplasms of nephron epithelial origin with the exception of cystic masses originating from the urinary tract (calyceal diverticulum or hydrocalyx) or resulting from a chronic infectious process (chronic abscess or hydatid cyst) (Fig. 8). Such nonepithelial benign cystic lesions commonly exhibit a thickened wall, which reflects the urothelial wall or a fibrous pseudocapsule, respectively, without suspicion of malignancy. Another benign entity called “localized cystic disease of the kidney” also should be ruled out before categorizing a multiloculated cystic renal mass. It represents clusters of benign cyst located within a portion of the kidney (Slywotzky and Bosniak 2001). Cystic renal tumors are surrounded by a capsule and contain enhancing septa in contrast to localized cystic disease in which a capsule is not present and individual cysts are separated by normal renal parenchyma.
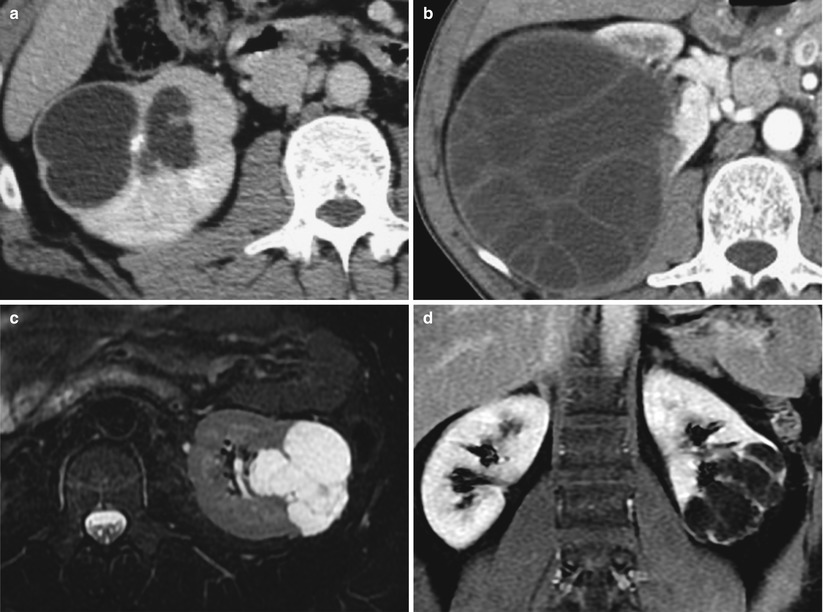

Fig. 8
(a, b) Benign cystic renal masses that should not be categorized in the Bosniak classification system. (a) Cystic lesion due to renal tuberculosis (dilated calyces and caseous necrosis). (b) Hydatid cyst presenting as a multiseptated cystic renal mass at CT. Contrast-enhanced CT (not shown) showed absence of wall or septa enhancement. (c, d) Localized cystic disease of the left kidney. Transverse T2-weighted image (c) and postcontrast T1-weighted image in a coronal plane (d) show cluster of renal cysts within the lower pole of the left kidney
Moreover, the discovery of a complex cystic lesion that exhibits a uniformly thickened wall with contrast enhancement in the clinical context of an acute event such as hemorrhage, acute infection, renal trauma, or recent renal surgery should lead to specific imaging workup (Fig. 9). In this particular case, follow-up CT until clinical recovery is usually indicated to separate inflammatory cystic lesions from complex cysts with suspicion of neoplasm that require further management using the Bosniak classification. Percutaneous aspiration and drainage can also be mandatory in cases consistent with acute abscesses, infected cysts, or diverticula.
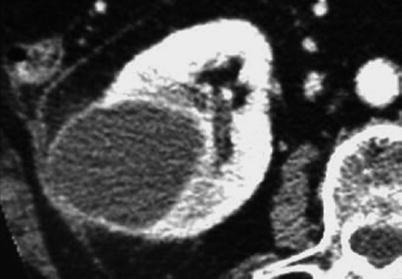

Fig. 9
Acute complicated benign cyst that needs specific imaging workup distinct from Bosniak classification scheme. Infected cyst with grossly uniform thick wall in a patient with acute pyelonephritis
4.2 Bosniak Categories (Fig. 10; Table 1)
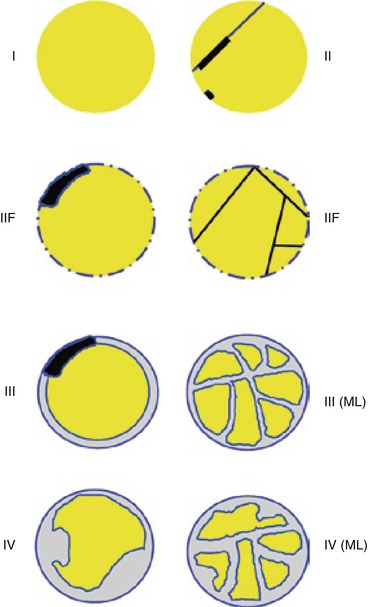
Fig. 10
Drawing of the different categories of renal cystic masses according to Bosniak classification. ML multilocular
Table 1
Bosniak classification system of renal cystic masses
Category | CT findings | Diagnosis |
|---|---|---|
Cat I | Water attenuation −5 to +15 HU) | Simple cyst |
Homogeneous | ||
Smooth margins without perceptible wall | ||
Lack of enhancement (variation < +10 HU) | ||
Cat II | Few thin septa (1 to 3 septa) no perceptible wall | Complicated cyst |
Fine calcification (wall or septum) | ||
Lack of enhancement (variation < +10 HU) or minimal (perceptible) enhancement of septa | ||
Cat IIF | More than a few thin septa | Complicated cyst |
Minimum wall thickening (≤1 mm) not measurable | Multilocular cyst | |
Thick or irregular calcification | Cystic tumor (cystic carcinoma or cystic nephroma) | |
Hyperdense cysta except for size ≥4 cm or intraparenchymal location | ||
Lack of enhancement (variation < +10 HU) or minimal enhancement (septa, wall) | ||
Cat III | Numerous thick septa | Complicated cyst |
Uniform grossly thick wall | Multilocular cyst | |
Slightly irregular thick wall | Cystic tumor (cystic carcinoma, cystic nephroma) | |
Thick or irregular calcification | ||
Enhancement (septa and/or wall) | ||
Cat IV | Grossly thick and irregular wall | Cystic carcinoma |
Mural nodules or solid tissue component | Pseudocystic necrotic RCC | |
Enhancement of the soft tissue elements |
The following paragraphs will describe the CT criteria that define each Bosniak category according to the original classification system. With the exception of their water characteristics (low signal intensity on T1 and bright hypersignal on T2-weighted images with lack of postcontrast enhancement) (Fig. 11) and the appearance of calcifications (not depicted at MRI), the following morphologic features can be also applied to categorize cystic lesions at MRI (Levy et al. 1994; Israel and Bosniak 2004). The hyperdense category II cyst also reveals specific signal findings at MRI compared to CT (refer to Sect. 5.2).
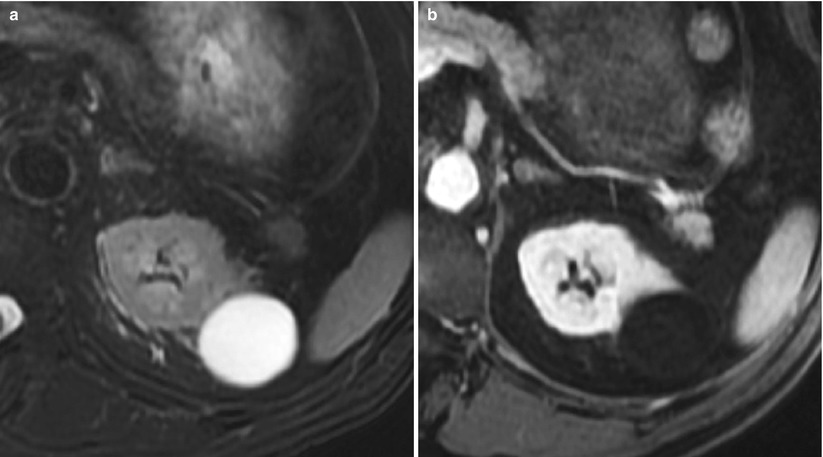

Fig. 11
(a, b) Simple cyst. Typical appearance at MRI. (a, b) T2-weighted (a) and postcontrast T1-weighted gradient echo (FSPGR with fat sat) axial image (b). The lesion typically fulfills the morphologic criteria of a simple cyst in the Bosniak classification along with water characteristics and the lack of postcontrast signal enhancement
4.2.1 Category I Lesions (Fig. 12)
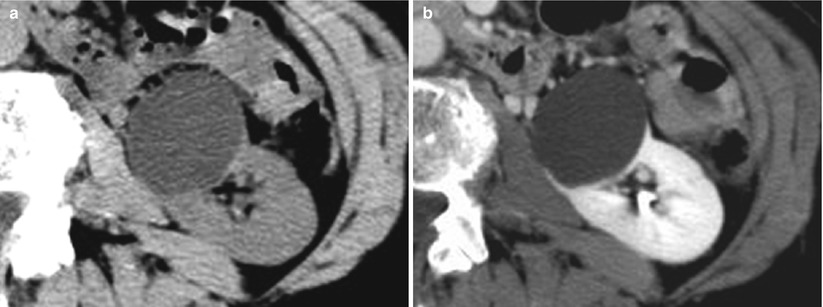
Fig. 12
(a, b) Category I cystic lesion. (a, b) Unenhanced (a) and contrast-enhanced (b) CT images show homogeneous “water” attenuation (8 HU), no enhancement (postcontrast attenuation 12 HU), homogeneous pattern with sharp margins, imperceptible wall and no internal septa or solid components, and absence of calcifications
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree