Renal Scintigraphy
Hossein Jadvar
LEARNING OBJECTIVES
1. Recognize various radiotracers employed in renal scintigraphy.
2. Name a number of renal diseases that may be evaluated effectively with scintigraphy.
3. Describe the utility and limitations of positron emission tomography with 18F-flurodeoxyglucose in the imaging evaluation of renal cell carcinoma.
Scintigraphy offers imaging-based diagnostic information on renal structure and function. Many single-photon radiotracers have long been in routine clinical use in renal scintigraphy. They are tailored to provide physiological information complementing the primarily structural-based imaging modalities, such as ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI). With the rapid expansion of positron emission tomography (PET), and more recently hybrid structural-functional imaging systems such as PET/CT, additional unprecedented opportunities have developed for quantitative imaging evaluation of renal diseases in both clinical and research arenas. In this chapter, we review the unique contribution of scintigraphy, including PET, in the imaging evaluation of renal structure and function. We first launch with a brief discussion of the common radiopharmaceuticals used in renal scintigraphy. Following a review of the normal patterns, we focus on various disease processes in which scintigraphy has been particularly contributory.
RADIOPHARMACEUTICALS
Technetium-99m diethylenetriaminepentaacetic acid
Technetium-99m diethylenetriaminepentaacetic acid (99mTc-DTPA) is the common agent for assessing glomerular filtration rate (GFR). The ideal agent for measuring GFR is cleared only by glomerular filtration and is not secreted or reabsorbed. 99mTc-DTPA satisfies the first requirement but has variable degrees of protein binding which deviate its kinetics from the ideal agent such as inulin. For a 20 mCi (740 MBq) dose, the radiation exposures to the kidneys and urinary bladder are 1.8 and 2.3 rads, respectively (1).
Iodine-131 orthoiodohippurate
The mechanism of iodine-131 orthoiodohippurate (131I-OIH) renal clearance is about 20% by GFR and 80% by tubular secretion. 131I-OIH is an acceptable alternative to para-aminohippuric acid (PAH) for determining renal plasma flow although its clearance is 15% lower than that of PAH. PAH is not entirely cleared by the kidneys with about 10% of arterial PAH remaining in the renal venous blood. Therefore, 131I-OIH measures effective renal plasma flow (ERPF). The tubular extraction efficiency of 131I-OIH is 90% and there is no hepatobiliary excretion. OIH may also be labeled with 123I that not only provides equivalent urinary kinetics as that for 131I label but also offers improved image quality due to typically larger administered dose in view of its more favorable radiation exposure. For a 300-uCi (11 MBq) dose of 131I-OIH, the radiation exposures to the kidneys and urinary bladder are 0.02 and 1.4 rads, respectively. Few drops of nonradioactive iodine (e.g., saturated solution of potassium iodide) orally minimize the thyroid uptake of free 131I (1).
Technetium-99m mercaptoacetyltriglycine
Technetium-99m mercaptoacetyltriglycine (99mTc-MAG3) has similar properties to OIH but has significant advantages of better image quality and less radiation exposure. The tubular extraction fraction of MAG3 is lower than OIH at about 60% to 70%. There is also about 3% hepatobiliary excretion which increases with renal insufficiency. Despite these features, however, MAG3 is a common agent used in the scintigraphic evaluation of renal function. For a 10-mCi (370 MBq) dose, the radiation exposures to the kidneys and urinary bladder are 0.15 and 4.4 rads, respectively (1).
Technetium-99m dimercaptosuccinic acid
Technetium-99m dimercaptosuccinic acid (99mTc-DMSA) localizes to renal cortex at high concentration and has slow urinary excretion rate. About 50% of the injected dose accumulates in the renal cortex at 1 h. The tracer is bound to the renal proximal tubular cells. Because of the high retention of DMSA in the renal cortex, it has become useful for the imaging of renal parenchyma. For a 6-mCi (11 MBq) dose, the radiation exposures to the kidneys and urinary balder are 3.78 and 0.42 rads, respectively (1).
Fluorine-18 fluorodeoxyglucose
Fluorodeoxyglucose (FDG) is the most common positron-labeled radiotracer in PET. 18F-labeled deoxyglucose is a modified form of glucose in which the hydroxyl group in the 2-position is replaced by the 18F positron emitter. FDG accumulates in cells in proportion to glucose metabolism. Cell membrane glucose transporters facilitate the transport of glucose and FDG across the cell membrane. Both glucose and FDG are phosphorylated in the 6-position by the hexokinase. The conversion of glucose-6-phosphate or FDG-6-phosphate back to glucose or FDG, respectively, is affected by the enzyme phosphatase. In most tissues, including cancer, there is little phosphatase activity. FDG-6-phosphate cannot undergo further conversions and, therefore, is trapped in the cell. FDG is excreted in the urine. The typical FDG dose is 0.144 mCi/kg (minimum 1 mCi and maximum 20 mCi). The urinary bladder wall receives the highest radiation dose from FDG (2,3). The radiation dose depends on the excretion rate, the varying size of the bladder, the bladder volume at the time of FDG administration, and an estimated bladder time-activity curve. For a typical 15 mCi FDG dose and voiding at 1 h after tracer injection, the average estimated absorbed radiation dose to the adult bladder wall is 3.3 rads (0.22 rads/mCi) (4). The doses to other organs are between 0.75 and 1.28 rads (0.050-0.085 rads/mCi) for an average organ dose of 1.0 rad (4).
NORMAL RENAL FUNCTION
GFR and ERPF may be assessed using dynamic quantitative nuclear imaging techniques. The GFR quantifies the amount of filtrate formed per minute (normal: 125 mL/min in adults). Only 20% of renal plasma flow is filtered through the semi-permeable membrane of the glomerulus. The filtrate is protein-free and nearly completely reabsorbed in the tubules. Filtration is maintained over a range of arterial pressures with autoregulation. The ideal agent for the determination of GFR is inulin which is only filtered but is neither secreted nor reabsorbed (1,5).
99mTc-DTPA is often employed to demonstrate renal perfusion and assess glomerular filtration, although 5% to 10% of injected DTPA is protein-bound and 5% remains in the kidneys at 4 h. A typical imaging protocol includes posterior 5-s flow images for 1 min followed by 1 min per frame images for 20 min. The GFR may be obtained using the Gates method that employs images of renal uptake during the second through third minute after DTPA administration. Regions of interest (ROIs) are drawn over the kidneys and background activity correction is applied. A standard dose is counted by the gamma camera for normalization. Depth photon attenuation correction is made based on a formula relating body weight and height. A split GFR can be obtained for each kidney which is not possible with the creatinine clearance method (1,5).
The ERPF (normal: 585 mL/min in adults) can be obtained by using OIH and MAG3 imaging (6). However, OIH has been largely replaced by MAG3 because of MAG3’s better imaging characteristics and dosimetry (due to radiolabeling with 99mTc). At present, MAG3 is the renal imaging agent of choice primarily because of the combined renal clearance of MAG3 by both filtration and tubular extraction which leads to the ability for obtaining relatively high-quality images even in patients with impaired renal function. The imaging protocol includes posterior 1-s images for 60 s (flow study) followed by 1-min images for 5 min and then 5-min images for 30 min. The relative tubular function may be obtained by drawing renal ROIs corrected for background activity. A renogram is constructed to depict the renal tracer uptake over time. The first portion of the renogram has a sharp upslope occurring in about 6 s following peak aortic activity (Phase I) representing perfusion followed by the extension to the peak value representing both renal perfusion and early renal clearance (Phase II). The next phase (Phase III) is downsloping and represents excretion. Normal perfusion of the kidneys is symmetric (50% ± 5%). The renogram peak occurs at about 2 to 3 min (vs. 3-5 min with DTPA) in normal adults and by 30 min, more than 70% of tracer is cleared and present in the urinary bladder (Fig. 13.1) (1,5).
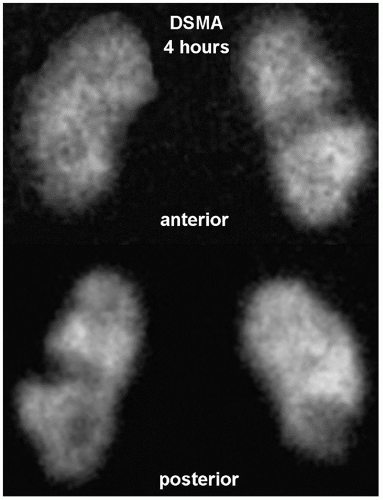
Renal cortical structure can be imaged with DMSA which correlates strongly with differential glomerular filtration and differential renal blood flow. Imaging is started 90 to 120 min after tracer administration, although images can be obtained at up to 4 h. Planar images are obtained in the anterior, posterior, left anterior (LAO)/right anterior (RAO), and right posterior oblique (RPO)/left posterior oblique (LPO) projections. Single-photon emission CT (SPECT) is also often obtained. A normal scan shows evenly distributed renal cortical uptake. Normal variations include dromedary hump (splenic impression on the left kidney), fetal lobulation, horseshoe kidney, crossed fused ectopia, and hypertrophied column of Bertin. The renal images also allow accurate assessment of the relative renal size, position, axis, and presence of cortical infarct (Fig. 13.2) (1,5).
CLINICAL APPLICATIONS
Renal failure
In renal failure, glomerular and tubular dysfunctions are reflected by abnormal renal scans and renograms. Renal uptake of MAG3 is prolonged with tubular tracer stasis and little or no excretion. It has been shown that in patients with acute renal failure, the demonstration of MAG3 renal activity more than hepatic activity at 1 to 3 min indicates likely recovery while when renal uptake is less than the hepatic uptake, dialysis may be needed (7). In chronic renal failure, there is diminished renal perfusion, cortical tracer extraction, and excretion. However, this imaging pattern is nonspecific and needs to be interpreted in the clinical context (1).
Renal infection
Acute pyelonephritis is associated with fever, flank pain, leukocytosis, and pyuria. Radiolabeled leukocyte (e.g., 111In-WBC) and 67Ga-citrate scans can help identify acute pyelonephritis. However, these methods have the drawbacks of extended imaging time (more than 24 h) and higher radiation exposure. Cortical imaging with DMSA is highly sensitive for detecting acute
pyelonephritis in the appropriate clinical setting (8). Acute pyelonephritis demonstrates segmental regions of decreased tracer uptake in oval, round, or wedge pattern. There may also be diffuse generalized decrease in renal uptake which in association with normal or slightly enlarged kidney is suspicious for an acute infectious process. The pathophysiologic basis for decline in DMSA cortical uptake in infection is related to diminished tracer delivery to the infected area and to direct infectious injury to the tubular cells compromising their function and tracer uptake. A wedge-shaped cortical defect with regional decrease in renal size is compatible with post-infectious scarring. Renal infarcts may also have a similar appearance (1,5).
pyelonephritis in the appropriate clinical setting (8). Acute pyelonephritis demonstrates segmental regions of decreased tracer uptake in oval, round, or wedge pattern. There may also be diffuse generalized decrease in renal uptake which in association with normal or slightly enlarged kidney is suspicious for an acute infectious process. The pathophysiologic basis for decline in DMSA cortical uptake in infection is related to diminished tracer delivery to the infected area and to direct infectious injury to the tubular cells compromising their function and tracer uptake. A wedge-shaped cortical defect with regional decrease in renal size is compatible with post-infectious scarring. Renal infarcts may also have a similar appearance (1,5).
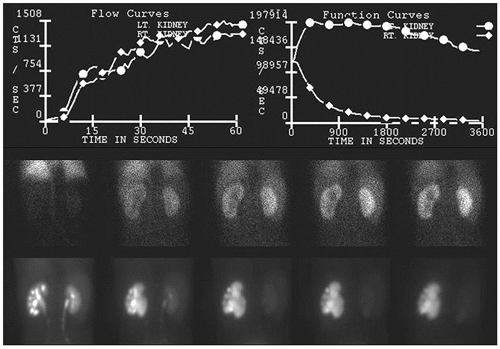 FIG. 13.3 • Abnormal 99mTc-MAG3 renogram following furosemide administration demonstrating left hydronephrosis and diminished left urinary clearance kinetics caused by ureteropelvic junction obstruction. 99mTc-MAG3, Technetium-99m mercaptoacetyltriglycine.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|