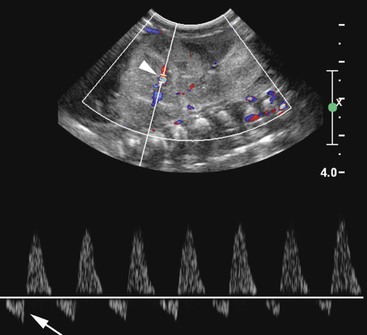
Giles Rottenberg, Allan C. Andi Renal transplantation was first performed in 1950 in the USA but with limited success due to the lack of immunosuppression. Initial success was experienced between identical twins where immunosuppression was not required. Cadaveric transplantation was only performed later when immunosuppression had been developed. There has been a recent increase in living donors—almost 50% in the USA and 30% in UK—due to the lack of availability of cadaveric kidneys. Recent development has seen the use of paired schemes or even more complicated pooled groups of donors sharing kidneys. ABO incompatibility is no longer a contraindication. Technique will vary amongst surgeons and is dependent upon the recipient’s vascular anatomy and presence of previous renal transplants. The transplant is usually sited in the right or left iliac fossa. The artery is anastomosed end to side onto the common or external iliac artery. If there are multiple arteries, the anastomosis may be onto a patch, although this is only possible from a cadaveric donor. The venous anatomy is usually performed as an end-to-side anastomosis onto the external iliac vein. The ureteric anastomosis is also variable according to the surgeon and may include an anti-reflux technique. The usual site for the anastomotic site is the dome of the bladder, although this may be altered if there is a large-capacity bladder in which the anastomosis may be lower. Ultrasound (US) remains the mainstay for imaging the kidney in both the short- and long-term management of the renal transplant, its advantages being its portability, cost and lack of ionising radiation and nephrotoxicity (see Fig. 37-1). The transplant kidney is ideally suited for US imaging; being superficially placed in the iliac fossa makes it possible for high-frequency probes to provide good-quality images. A full examination of the transplant relies upon a combination of grey scale, and Doppler US. The graft length should be measured and evaluated for evidence of hydronephrosis, calculi or focal abnormality. The peri-renal tissues should be evaluated for fluid collections, and the bladder examined for wall-thickening, calculi or tumour. The orientation of the kidney can vary depending on surgical technique and should be established in the axial plane so that true longitudinal images can be obtained. The presence of ureteric stents should be noted and reported. The bladder should be evaluated both before and after micturition, particularly if there is hydronephrosis or urinary tract infection. Doppler US should routinely be used. Colour flow and spectral Doppler evaluation of the intra-renal blood flow and the extra-renal vessels should be undertaken if there is a history of hypertension. The spectral pattern of the normal renal transplant artery is similar to that in the native renal artery. Various measurements of the Doppler trace can be made, although there is limited clinical value in performing extensive measurements as they tend not to be specific enough to differentiate between rejection and acute tubular necrosis (ATN). The normal trace is a low-resistance circulation (ski slope pattern) with the diastolic flow measuring approximately a third of the systolic velocity. A reduction in the diastolic velocity is usually a sign of a pathological process. The resistive index (RI) is a useful simple measurement for qualitative analysis, and is useful for communicating with clinicians. RI is measured by RIs exceeding 0.8 in the kidney transplant allograft have been defined as abnormal; however, multiple researchers have documented RI’s lack of specificity considering the complex interaction between renal vascular resistance and compliance. Therefore, clinical assessment and associated sonographic findings remain integral in the assessment of graft dysfunction.1,2 In addition to intra-renal measurement of Doppler flow, a systematic assessment of colour flow in all regions of the kidney should be performed to assess differential blood flow. Although evaluation of colour flow is subjective, it is a useful tool and reassuring when normal. This is particularly important when multiple renal arteries are present at the time of transplant. It is important to evaluate the native kidneys if the patient is being investigated for recurrent urinary tract infection, as these may be a source of sepsis or stones. Similarly, the investigation of haematuria post-transplant must include an assessment of the native kidneys for hydronephrosis or renal mass. Computed tomography (CT) and magnetic resonance imaging (MRI) are both useful in the further evaluation of transplant complications where US cannot fully demonstrate an abnormality due to overlying bowel gas or when patients become systemically unwell and US is unable to demonstrate an abnormality. CT can be used to confirm vascular complications which have been detected by US, or where US has not been able to sufficiently exclude a vascular abnormality. A dedicated renal CT angiographic protocol with multiplanar reconstruction or a biphasic examination may be required to answer these questions. Magnetic resonance (MR) angiography is an evolving technique that can be used to evaluate the transplant artery and vein. It is used predominantly when US evaluation is inconclusive. There are two main techniques of MR angiography—non-contrast and contrast-enhanced. Contrast-enhanced MR angiography relies upon the administration of gadolinium, which was widely used in the past in patients with renal dysfunction in preference to iodinated contrast agents. It is now contraindicated in patients with renal failure or severe dysfunction (i.e. with an estimated glomerular filtration rate of less than 30) due to the risk of nephrogenic systemic fibrosis. Contrast-enhanced MR angiography also relies upon a coronal three-dimensional (3D) fast multiplanar gradient-echo breath-hold sequence. This is performed after a timing bolus of contrast medium. Many techniques are used to perform unenhanced MR angiography, the most frequently used being the time-of-flight (TOF) or phase-contrast method. TOF exploits the inflow effect of blood protons and uses flow-compensated 2D or 3D gradient-echo sequences. Phase contrast is independent of direction but is motion susceptible and time-consuming. Turbulent flow can cause loss of signal and may result in overestimation of narrowing. Newer techniques which appear to offer significant improvement in image quality have been recently introduced. Steady-state free precession (SSFP) with or without arterial spin labelling can provide high-resolution images of blood vessels, including renal arteries, without the use of contrast agents. It relies upon a gradient-echo-based sequence that maintains steady-state longitudinal and transverse magnetisation by applying serial equidistant radiofrequency pulses. SSFP enables a short acquisition time and produces high signal-to-noise images which are independent of flow and direction. The images are susceptible to field heterogeneity. Compared with CT angiography, MR angiography has a number of disadvantages, which include an increased time of imaging, a limited range and a range of complex artefacts. Conventional angiography is usually reserved as a prelude to intervention, or in cases where US and/or CT are unable to exclude or define an abnormality. Cystography may be required in the evaluation of bladder abnormality immediately after surgery to evaluate bladder injury, or at a later date to investigate reflux following recurrent pain or infection (see Fig. 37-2). Transplant thrombosis, either arterial or venous, is uncommon, occurring in 1–5% of transplants,3 usually in the early postoperative period, and often complicating severe acute rejection, tubular necrosis and torsion or angulation of the surgical anastomosis. The assessment of renal venous flow can readily be obtained by Doppler US (see Fig. 37-3). In renal vein thrombosis, reverse diastolic flow within both main and intra-renal arteries is evident at spectral Doppler US. In renal artery thrombosis, flow in both main and intra-renal arteries is completely absent at colour Doppler. In intra-renal artery segmental infarction, a focal area of decreased echogenicity, typically wedge-shaped with associated diminished vascularity, is evident at colour Doppler. Confirmation, if necessary, can be achieved with gadolinium-enhanced MR, CT or selective angiography. Surgical thrombectomy with arterial or venous repair is typically performed once acute thrombosis has been confirmed; nephrectomy may be required if the kidney is non-viable. The role of endovascular management of graft thrombosis with mechanical and chemical thrombolytic techniques is currently evolving and may also be considered.4,5 Rejection, occurring as early as 1 week after transplantation, and peaking at 1–3 weeks, may be classified as either acute rejection or accelerated acute rejection. Whilst patients usually remain asymptomatic with deteriorating renal function, a myriad of US appearances may be demonstrated. The graft may be oedematous, with swelling of the medullary pyramids, reduced cortical echotexture and loss of normal corticomedullary differentiation; in severe cases, there may be complete absence of diastolic flow or flow reversal with associated elevation of the RI. Doppler findings are not specific for acute rejection and may be seen in ATN, renal vein thrombosis or drug toxicity. In patients with an appropriate clinical history and supporting sonographic findings, empirical immunosuppressive therapy may be commenced but percutaneous biopsy remains the gold standard for the diagnosis of rejection. ATN is the commonest cause of early failure of function following cadaveric transplantation. It relates to prolonged cold ischaemic times in cadaveric grafts when compared with living grafts. Clinical differentiation of ATN from acute rejection occurring in the first 10 days can be difficult. Similarly, the spectrum of sonographic appearances may vary from normal to those seen in acute rejection. As previously mentioned in relation to acute rejection, RIs are typically elevated; however, clinically significant ATN may also be demonstrated in conjunction with a normal RI. Serial radionuclide imaging studies have proven reliable in differentiating the entities by the time course of the findings. However, histological confirmation remains the standard and biopsy is frequently required. Postoperative pelvic collections following transplantation are common, and may be due to urinoma, lymphocele, haematoma or a seroma (see Figs. 37-4–37-7). Differentiation can often be made clinically according to symptoms and time since surgery. Urinoma formation usually occurs soon after surgery, whereas lymphocele formation usually develops at a slightly later stage.
Renal Transplantation
Imaging
History of Transplant
Surgical Technique
Technique of Examination
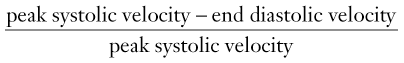
Vascular Complications: Early3
Infarction
Acute Rejection
Acute Tubular Necrosis
Non-vascular Complications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Renal Transplantation
Chapter 37