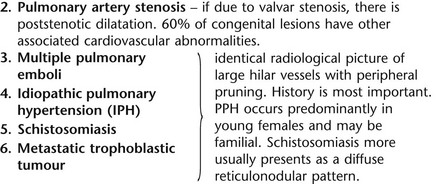
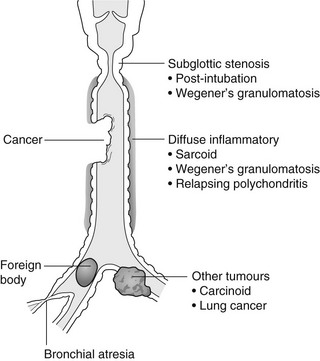
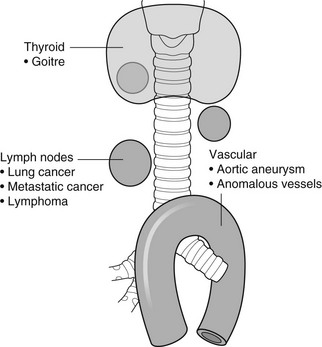
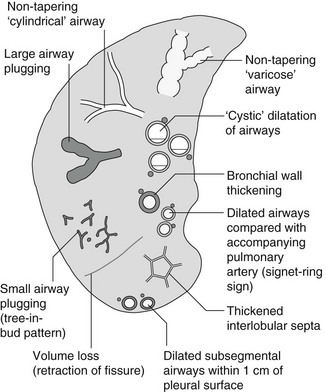
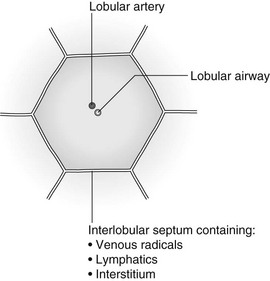
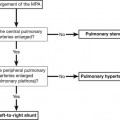
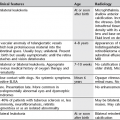
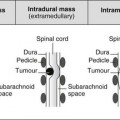
4 4.1 Unilateral hypertransradiant hemithorax 1. Compensatory hyperexpansion – e.g. following lobectomy (look for rib defects/bronchial sutures indicating previous surgery) or lobar collapse. 2. Airway obstruction – air trapping on expiration results in increased lung volume and shift of the mediastinum to the contralateral side. 3. Unilateral bullae – vessels are absent rather than attenuated. May mimic pneumothorax. 4. Swyer–James (McLeod) syndrome – the late sequela of bronchiolitis in childhood (usually viral but non-viral organisms also implicated). Lung volume on affected side is either normal or slightly reduced but, importantly, there is air trapping on expiration. Ipsilateral hilar vessels are small. CT not infrequently shows bilateral disease with mosaic attenuation and bronchiectasis. 5. Congenital lobar emphysema – one-third present at birth. Marked overinflation of a lobe (most commonly left upper lobe followed by right upper lobe or right middle lobe). The ipsilateral lobes are compressed and there may be mediastinal displacement to the contralateral side. 4.2 Bilateral hypertransradiant hemithoraces 1. Emphysema – with large central pulmonary arteries and peripheral arterial pruning ± bullae; centrilobular emphysema typically in mid/upper zones whereas panacinar emphysema commonly affects lower zones. 2. Asthma – during an acute episode or with chronic disease with ‘fixed’ airflow obstruction due to airway remodelling. 3. Acute bronchiolitis – particularly affects children in the first year of life. Overexpansion is due to small airways (bronchiolar) obstruction. May be associated with large airway mucosal thickening leading to bronchial wall thickening on plain radiography. Collapse and consolidation are not primary features of bronchiolitis. 4. Tracheal, laryngeal or bilateral bronchial stenoses (see 4.3). Engelke, C., Schaefer-Prokop, C., Schirg, E., et al. High-resolution CT and CT angiography of peripheral pulmonary vascular disorders. RadioGraphics. 2002; 22:739–764. Frazier, A. A., Galvin, J. R., Franks, T. J., et al. Pulmonary vasculature: hypertension and infarction. RadioGraphics. 2000; 20:491–524. 4.3 Tracheal/bronchial narrowing, stenosis or occlusion 1. Foreign body – air trapping is more common than atelectasis. The lower lobe is most frequently affected. The foreign body may be opaque. The column of air within the bronchus may be discontinuous (the ‘interrupted bronchus sign’). 2. Mucus plug – e.g. allergic bronchopulmonary aspergillosis, asthma. 3. Misplaced endotracheal tube. 1. Tracheal/lung cancer – narrowing ± irregularity. 2. Other tumours – e.g. carcinoid tumour – usually a smooth, rounded filling defect. Main bulk of tumour may lie outside the lumen of the airway. 3. Inflammation/fibrosis – e.g. sarcoidosis, Wegener’s granulomatosis, post-tuberculous, relapsing polychondritis. 4. Bronchial atresia – most commonly affecting the apicoposterior segment of the left upper lobe. 5. Tracheobronchomalacia – excessive reduction (> 70% luminal calibre) in the calibre of the trachea/central airways at end-expiration. Diagnosis made on CT performed at residual volume or during dynamic expiration. 2. Mediastinal tumours – smooth, eccentric narrowing. 5. Anomalous origin of left pulmonary artery from right pulmonary artery – producing compression of the right main bronchus as it passes over it, between the trachea and oesophagus to reach the left hilum. PA CXR shows the right side of the trachea to be indented and the vessel is seen end-on between the trachea and oesophagus on the lateral view. Chung, J. H., Kanne, J. P., Gilman, M. D. Structured review article – CT of diffuse tracheal diseases. AJR Am J Roentgenol. 2011; 196:W240–W246. Prince, J. S., Duhamel, D. R., Levin, D. L., et al, Non-neoplastic lesions of the tracheobronchial wall: radiologic findings with bronchoscopic correlation. RadioGraphics. 2002;(Suppl):S215–S230. 4.4 Increased density of one hemithorax 1. Consolidation/air-space opacification – indicating the replacement of air from the air spaces by exudate or other disease process (e.g. tumour, blood, oedema). An air bronchogram/bronchiologram may be present. Vascular margins and airway wall are obscured. 2. Pleural effusion – in the supine position, an uncomplicated effusion gravitates to the dependent part of the chest, producing a generalized increased density ± an apical ‘cap’ of fluid on CXR. Note that pulmonary vessels will be visible through the increased density (cf. Consolidation/air-space opacification). Erect or decubitus CXRs may confirm the diagnosis. 3. Malignant pleural mesothelioma – often associated with a pleural effusion which obscures the tumour ± calcified pleural plaques (more commonly demonstrated at CT). Encasement of the lung limits mediastinal shift. (NB. Affected hemithorax may even be smaller.) 1. Large pleural effusion (q.v.) – NB. A large effusion with no mediastinal shift indicates significant lung collapse (and, hence, central obstruction) or relative ‘fixation’ of the mediastinum (e.g. caused by malignant pleural mesothelioma). 2. Diaphragmatic hernia – on the right side with herniated liver; on the left side the hemithorax is not usually opaque because of air within the herniated bowel. The left hemithorax may be opaque in the early neonatal period when air has not yet had time to reach the herniated bowel. 3. Lymphangitis carcinomatosa – bilateral and symmetrical infiltration is most common; unilateral lymphangitis occurs more often in patients with lung cancer. Linear and nodular opacities ± ipsilateral hilar and mediastinal lymph-node enlargement. Septal lines. Pleural effusions are common. 4. Pulmonary agenesis and hypoplasia – usually asymptomatic. Absent or hypoplastic pulmonary artery. 4.5 Pulmonary air cysts Cysts can appear during the first 2 weeks of the pneumonia and may resolve over several months. 1. Langerhans’ cell histiocytosis (LCH) – cysts, sometimes with bizarre (i.e. non-circular) outlines, in mid/upper zones. In ‘early’ disease multiple nodules, which then cavitate. Relative sparing of lower zones and medial tips of middle lobe and lingula. Pulmonary LCH (but not other forms of LCH) is strongly linked to cigarette smoking. 2. Lymphangioleiomyomatosis (LAM) – exclusively in female subjects of child-bearing age and related to mutation of TSC1 (chromosome 9). Smooth-muscle proliferation around vessels, lymphatics and airways. Cysts are a characteristic finding and more uniform in size than in LCH. No zonal predilection (cf. LCH). 3. Tuberose sclerosis (TSC) – neurocutaneous disorder associated with mutations of TSC1 and TSC2 genes. Pathology of lung disease in TSC almost identical to LAM. 4. Neurofibromatosis – cystic lung disease and interstitial fibrosis are reported but some doubt exists and changes maybe simply represent smoking-related interstitial lung disease. 5. Birt–Hogg–Dubé syndrome – autosomal dominant multisystem disorder characterized by pulmonary cysts, cutaneous fibrofolliculomas and increased risk of renal tumours. Recurrent pneumothoraces due to lung involvement. 6. Lymphoid interstitial pneumonia – rarely idiopathic; usually occurs in context of dysproteinaemias, HIV infection and connective tissue disorders (in particular rheumatoid arthritis, Sjögren’s syndrome). Mechanism of cyst formation is uncertain but may be caused by partial obstruction of small airways. 7. Hypersensitivity pneumonitis – possibly related to lymphocytic interstitial pulmonary infiltrate in subacute phase. 8. End-stage fibrotic diffuse interstitial lung diseases – e.g. idiopathic pulmonary fibrosis, sarcoidosis, chronic hypersensitivity pneumonitis. Biyyam, D. R., Chapman, T., Ferguson, M. R., et al. Congenital lung abnormalities: embryologic features, prenatal diagnosis, and postnatal radiologic–pathologic correlation. RadioGraphics. 2010; 30:1721–1738. Seaman, D. M., Meyer, C. A., Gilman, M. D., McCormack, F. X. Pictorial essay: diffuse cystic lung disease at high-resolution CT. AJR Am J Roentgenol. 2011; 196:1305–1311. Silva, C. I. Diffuse lung cysts in lymphoid interstitial pneumonias: high-resolution CT and pathologic findings. J Thorac Imaging. 2006; 21:241–244. 4.6 Non-resolving or recurrent consolidation 1. Bronchial obstruction – e.g. caused by a tumour or foreign body. 2. Inappropriate antimicrobial therapy – e.g. tuberculosis, Klebsiella and fungal infection. 3. Malignancy – adenocarcinoma, lymphoma. 4. Recurrent aspiration – secondary to a pharyngeal pouch, achalasia, systemic sclerosis, hiatus hernia, ‘H’ type tracheo-oesophageal fistula, paralytic/neuromuscular disorders, chronic sinusitis. 5. Pre-existing lung pathology – e.g. bronchiectasis (see 4.10). 6. Impaired immunity – e.g. prolonged corticosteroid or other immunosuppressive therapy, immunoglobulin deficiency, diabetes, cachexia, HIV-related. 4.7 Consolidation with an enlarged hilum 1. Primary tuberculosis – lymph-node enlargement is unilateral in 80% and involves the hilar (60%), or combined hilar and paratracheal (40%) nodes. 3. Mycoplasma pneumonia – lymph-node enlargement is common in children but rare in adults. May be unilateral or bilateral. 4. Primary histoplasmosis – in endemic areas. Hilar lymph-node enlargement is common, particularly in children. During healing, lymph nodes calcify and may cause bronchial obstruction, thereby initiating distal infection. 5. Coccidioidomycosis – in endemic areas. The pneumonic type consists of predominantly lower lobe consolidation that is frequently associated with hilar lymph-node enlargement. 4.8 Pneumonia involving part or the whole of one lobe 1. Streptococcal pneumonia – the commonest cause. Usually unilobar. Cavitation is rare. Pleural effusion is uncommon. Little or no collapse. 2. Klebsiella pneumonia – often multilobar involvement. High propensity for cavitation and lobar enlargement. 3. Staphylococcal pneumonia – especially in children. 40–60% of children develop pneumatocoeles. Effusion (empyema) and pneumothorax are also common. Bronchopleural fistula may develop. No lobar predilection. 4. Tuberculosis – in primary or postprimary tuberculosis, but more common in the former. Associated collapse is common. The right lung is affected more often than the left, and primary tuberculosis has a predilection for the anterior segment of the upper lobes or the medial segment of the middle lobe. 5. Streptococcus pyogenes pneumonia – affects the lower lobes predominantly. Often associated with pleural effusion/empyema. 4.9 Consolidation with bulging of fissures Homogeneous or inhomogeneous air-space opacification with bulging of the bounding fissures. 1. Infection with abundant exudates – pneumonia caused by Klebsiella pneumoniae (Friedländer’s pneumonia), Streptococcus pneumoniae, Mycobacterium tuberculosis and Yersinia pestis (plague pneumonia). 2. Abscess – when an area of consolidation breaks down. Organisms that commonly produce abscesses are Staphylococcus aureus, Klebsiella spp. and other Gram-negative organisms. 4.10 Bronchiectasis 1. Following childhood infections – e.g. secondary to measles and pertussis. Less common cause in the antibiotic era in developed countries but continues to be an important factor in developing countries. 2. Secondary to bronchial obstruction – foreign body, neoplasm, broncholithiasis or bronchial stenosis. 4. Congenital/genetic anomalies (a) Kartagener’s syndrome – bronchiectasis with immobile cilia, dextrocardia and absent frontal sinuses. 5% of patients with dextrocardia will eventually develop bronchiectasis. (b) Williams–Campbell syndrome – bronchial cartilage deficiency. (c) Mounier–Kuhn syndrome – tracheobronchomegaly. (d) α1-Antitrypsin deficiency. 5. Collagen vascular diseases – particularly rheumatoid arthritis, Sjögren’s syndrome. 6. Gastrointestinal disorders – ulcerative colitis, coeliac disease. 7. Immunological – allergic bronchopulmonary aspergillosis, following solid-organ (heart/lung) or bone marrow transplantation. Dodd, J. D., Souza, C. A., Müller, N. L. Conventional high-resolution CT versus helical high-resolution MDCT in the detection of bronchiectasis. AJR Am J Roentgenol. 2006; 187:414–420. Hansell, D. M., Lynch, D. A., McAdams, H. P., Bankier, A. A., Diseases of the airways diseases. Imaging of diseases of the chest. 5th ed. Elsevier Mosby, Philadelphia, PA, 2010. McGuinness, G., Naidich, D. P. CT of airways disease and bronchiectasis. Radiol Clin North Am. 2002; 40(1):1–19. 4.11 Air-space opacification Note that any of the following may be unilateral and, in some instances, confined to a single lobe. 2. Infection – see also 4.7–4.9 (d) Influenza – particularly in patients with mitral stenosis or who are pregnant. (e) Chicken pox (and other viral pneumonias) – may be confluent in the central areas of the lungs. ± Hilar lymph-node enlargement. 3. Diffuse pulmonary haemorrhage – e.g. idiopathic pulmonary haemosiderosis, anti-glomerular basement disease, microscopic polyangiitis, systemic lupus erythematosus, Behçet’s syndrome, Wegener’s granulomatosis, contusion, bleeding diatheses, Goodpasture’s syndrome, idiopathic pulmonary haemosiderosis (in the acute stage), pulmonary infarction. 4. Malignancy – adenocarcinoma, lymphoma. 5. Sarcoidosis* – called ‘air-space’ sarcoidosis and occurring in up to 20%. Air-space pattern is due to thickened interstitium and filling of air spaces by macrophages and granulomatous infiltration. 6. Eosinophilic lung disease – chronic eosinophilic pneumonia is characteristically non-segmental, in the upper zones and paralleling the chest wall. 7. Organizing pneumonia – may be cryptogenic or as a response to other ‘insult’ (e.g. infection, drug toxicity, connective tissue disease). Typically, multifocal air-space opacities in periphery of mid/lower zones. Occasionally a single focus may be present. A characteristic perilobular distribution is seen in some patients. Another pattern is the so-called ‘reverse halo’ or Atoll sign (a ring of consolidation surrounding central ground-glass opacification). 4.12 Pulmonary oedema 1. Fluid overload – excess i.v. fluids, renal failure and excess hypertonic fluids, e.g. contrast media. 2. Cerebral disease – cerebrovascular accident, head injury or raised intracranial pressure. 3. Near drowning – radiologically no significant radiological differences between freshwater and seawater drowning. 4. Aspiration (Mendelson’s syndrome). 5. Radiotherapy – several weeks following treatment. Ultimately has a characteristic straight edge as fibrosis ensues. 6. Rapid re-expansion of lung following thoracocentesis. 7. Liver disease and other causes of hypoproteinaemia. 8. Transfusion-related acute lung injury (TRALI) – commonest cause of transfusion-related mortality in UK and USA. Onset of oedema is either during transfusion or within 1–2 hours. (a) Those which induce cardiac arrhythmias or depress myocardial contractility (contrast media can induce arrhythmias, alter capillary wall permeability and produce a hyperosmolar load). (b) Those which alter pulmonary capillary wall permeability, e.g. overdoses of heroin, morphine, methadone, cocaine, ‘crack’, dextropropoxyphene and aspirin. Hydrochlorothiazide, phenylbutazone, aspirin and nitrofurantoin can cause oedema as an idiosyncratic response; interleukin-2 and tumour necrosis factor may cause increased permeability by an unknown pathophysiological process. 11. Mediastinal tumours – producing venous or lymphatic obstruction. 12. Acute respiratory distress syndrome – may be primary (e.g. caused by severe pneumonia, aspiration) or secondary (e.g. following non-thoracic sepsis or trauma); CXR may be normal in first 24 hours but progressive widespread opacification with onset of interstitial and then frank intra-alveolar leak of oedema and haemorrhagic fluid. 13. High altitude (acute mountain sickness) – following rapid ascent to > 3000 metres. Asrani, A. Urgent findings in portable chest radiography. AJR Am J Roentgenol. 2011; 196:WS37–WS46. Gluecker, T., Capasso, P., Schnider, P., et al. Clinical and radiologic features of pulmonary edema. RadioGraphics. 1999; 19:1507–1531. Desai, S. R., Wells, A. U., Suntharalingam, G., et al. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary injury: a comparative CT study. Radiology. 2001; 218:689–693. Desai, S. R. Acute respiratory distress syndrome: imaging the injured lung. Clin Radiol. 2002; 57:8–17. 4.13 Unilateral pulmonary oedema Oedema on the side opposite a lung with a perfusion defect. 4.14 Septal (Kerley B) lines 1. Lymphangitis carcinomatosa/lymphomatosa – most often caused by lymphatic infiltration in patients with cancer of the lung, breast, stomach and pancreas or lymphoma. Nodular thickening (typically bilateral) of interlobular septa is the characteristic finding on CT. 2. Pneumoconioses – surrounding tissues may contain a heavy metal, e.g. tin, which contributes to the density. 3. Sarcoidosis* – septal lines are uncommon. 4. Idiopathic bronchiectasis – thickened interlobular septa are a feature in around one-third of patients (see 4.10). 5. Erdeim–Chester disease – infiltration of pulmonary interstitium by histiocytes of non-Langerhans’ type. Primarily a bone disorder but extraskeletal involvement in around 50%. Lung involvement in around one-third of patients. On chest radiography, reticulonodular infiltrate in mid/upper zones. On CT, smooth thickening of interlobular septa is characteristic, associated with ground-glass opacification and centrilobular nodules. 6. Diffuse pulmonary haemorrhage – with recurrent episodes of bleeding, thickened interlobular septa may be seen on CT (see 4.11). 7. Diffuse pulmonary lymphangiomatosis – proliferation of lymphatic channels in pleura, interlobular septa and peribronchovascular connective tissue. 8. Congenital lymphangiectasia – abnormal dilatation of lymphatic vessels. (NB. No increase in number of lymphatics; cf. lymphangiomatosis.) May be associated with extrathoracic congenital anomalies (e.g. renal, cardiac). Usually fatal. 9. Alveolar proteinosis – smooth thickening of interlobular (and intralobular) septa in geographical areas of ground-glass infiltration (i.e. the ‘crazy-paving’ pattern). Infiltration of air spaces and interstitium and air spaces by PAS-positive macrophages. 4.15 Reticular pattern (with or without honeycombing) 1. Idiopathic pulmonary fibrosis (IPF) – most common idiopathic interstitial pneumonia. Associated with the histological/radiological pattern of UIP. Typical patient is male, aged 50–60 years and complaining of progressive dyspnoea. On CXR there is a reticular pattern in mid/lower zones. Predominant basal and subpleural reticular pattern with honeycombing is the characteristic finding on CT. Ancillary findings include ground-glass opacification (less extensive than reticular pattern), traction bronchiectasis/bronchiolectasis and mediastinal lymph-node enlargement. Atypical HRCT findings in around 30–50%. Coexistent emphysema in upper zones (associated with preserved lung volumes). Increased incidence of lung cancer. 2. Connective tissue diseases (CTDs) – fibrotic lung disease is common and most frequent cause of death. Variable patterns of interstitial lung disease including UIP, NSIP, lymphoid interstitial pneumonia (LIP; see also 4.5) and diffuse alveolar damage. Variable prevalence in different CTDs: UIP pattern more prevalent than NSIP in rheumatoid arthritis; NSIP pattern more prevalent in systemic sclerosis and polymyositis/dermatomyositis; LIP most prevalent in rheumatoid arthritis and Sjögren’s syndrome. (a) Asbestosis: basal and subpleural fibrosis, almost indistinguishable from IPF on histopathology and radiology although fibrosis may be coarser in asbestosis than IPF and pleural abnormalities absent in IPF. Long latency period (> 20 years) following exposure. (b) Hard metal pneumoconiosis: exposure to alloys of tungsten, carbon and cobalt ± other metals. (c) Paraquat poisoning: late phase of poisoning associated with pulmonary fibrosis. 4. Sarcoidosis* – archetypal granulomatous fibrotic diffuse interstitial lung disease. Typically associated with symmetrical, bronchocentric reticular pattern in upper zones. Calcified mediastinal and hilar lymph nodes. 5. Chronic hypersensitivity pneumonitis – secondary to repeated exposure to a potentially wide variety of particulate (1–5 µm diameter) organic antigens originating from animals, plants, drugs or bacteria/fungi. A reticular pattern ± honeycombing, ground-glass opacification ± traction bronchiectasis/bronchiolectasis and lobular areas of apparently ‘spared’ lung are characteristic on CT. 6. Cystic lung diseases – because of the effects of anatomical superimposition on chest radiographs, the multiple cysts in disorders such as Langerhans’ cell histiocytosis, lymphangioleiomyomatosis and tuberous sclerosis can manifest as a reticular pattern (see also 4.5). Relative preservation of lung volumes. 7. Drug-induced lung disease – e.g. caused by nitrofurantoin, busulphan, cyclophosphamide, bleomycin and melphalan. 8. Bone marrow transplantation – airways disease (constrictive obliterative bronchiolitis) and upper zone fibrosis associated with recurrent small pneumothoraces. 9. Miscellaneous causes of diffuse lung disease Seen in α1-antitrypsin deficiency-related emphysema; seen in the lower zones. Dixon, S., Benamore, R. The idiopathic interstitial pneumonias: understanding key radiological features. Clin Radiol. 2010; 65:823–831. Raghu, G., Collard, H. R., Egan, J. J., et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis evidence-based guideline for diagnosis and management. Am J Respir Crit Care Med. 2011; 183:788–824. Wells, A. U., Hirani, N. Interstitial lung disease guideline. Thorax. 2008; 63(Suppl V):v1–v58. 4.16a Multiple small (‘pin-point’) micronodules Must be of very high atomic number to be rendered visible on plain chest radiography. 1. Post lymphangiography – iodized oil emboli. Contrast medium may be visible at the site of termination of the thoracic duct. 2. Silicosis* – usually larger than pin-point but can be very dense, especially in gold miners. 3. Stannosis – inhalation of tin oxide. Even distribution throughout the lungs. With Kerley A and B lines. 4. Barytosis – inhalation of barytes. Very dense, discrete opacities. Generalized distribution but bases and apices usually spared. 5. Limestone and marble workers – inhalation of calcium. 6. Alveolar microlithiasis – familial disorder. Lung detail obscured by miliary calcifications. Few symptoms but may progress to cor pulmonale. Pleura, heart and diaphragm may be seen as ‘negative’ shadows. 4.16b Multiple micronodules (0.5–2 mm)
Respiratory tract
Lung
With overexpansion of the lungs
In the lumen
In the wall
Outside the wall
With an undisplaced mediastinum
With mediastinal displacement away from the dense hemithorax
With mediastinal displacement towards the dense hemithorax
Postinfective
Neoplastic
Diffuse lung diseases
Primary pneumonias
Causes of bronchiectasis
Less frequent
Causes of air-space opacification
Non-cardiogenic pulmonary oedema
Pulmonary oedema on the same side as a pre-existing abnormality
Pulmonary oedema on the opposite side to a pre-existing abnormality
Lymphatic/interstitial infiltration
Reticular pattern – diffuse interstitial lung diseases
Reticular pattern – due to parenchymal destruction leaving ‘remnant’ interlobular septa
Soft-tissue or ground-glass attenuation
Respiratory tract