Monitoring the response of tumors to treatment has become an integral component of oncologic imaging. Imaging studies play a vital role in objective assessment by quantifying tumor response to a variety of physical and pharmaceutical treatments. Traditionally, therapeutic response has been assessed by conventional methods that involve serial tumor burden measurements according to the World Health Organization (WHO) and Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Computed tomography (CT) and magnetic resonance imaging (MRI) provide reliable and reproducible anatomic data to determine changes in tumor burden through treatment. However, the management of many tumors has extended beyond traditional chemotherapy and novel targeted therapies (such as antiangiogenic agents) have been introduced. With respect to targeted therapy, changes in tumor dimension may not be evident in the early course of treatment and measurement alone has been shown to have limitations. At the same time, to maximize the effectiveness of care, it is important to differentiate between responders and nonresponders as early as possible. Hence, monitoring effects from these expensive targeted therapies has introduced new expectations from imaging techniques. Conventional measurement-based methods are still widely used, but new techniques are being increasingly adopted. Emerging techniques such as positron emission tomography (PET or PET/CT) can be used to look at changes in tumor metabolism. There has been growing interest in the use of tumor viability measurement, dynamic contrast-enhanced imaging, or perfusion imaging to assess tumor vascular microenvironment and early antiangiogenic effects. Other advances in MRI such as diffusion weighted imaging (DWI) are also evolving as biomarkers of cellular integrity. Despite availability of various imaging techniques, it is challenging to determine the most appropriate image biomarker to serve as a surrogate end point of treatment response. An ideal imaging biomarker should be noninvasive, objectively quantitative, reproducible, readily available, validated, and easy to implement in the clinical setting. This chapter discusses various established and emerging and evolving imaging biomarkers, the criteria of response evaluation, and their challenges.
Response Criteria Based on Tumor Burden Measurement
World Health Organization Criteria
Response evaluation with diagnostic imaging has continuously evolved since the late 1970s. It has been generally accepted that a decrease in tumor size correlates with treatment effect. In view of this, the WHO first published a set of tumor response criteria in 1981, which was mainly used in clinical trials in which tumor response was the primary end point. The WHO criteria for tumor burden assessment were based on summing bidimensional, or two-dimensional (2D), measurements. The WHO criteria received wide acceptance, but were soon realized to have potential pitfalls such as no information on minimum lesion size or the number of lesions to be selected in patients with multiple lesions, the type of imaging modality that should be used, and lack of clarity among some definitions of response criteria. For example, a 25% increase in the product of 2D diameters (progressive disease) was defined by some investigators as increase in any one lesion and by others as increase in the sum of all selected lesions.
It was observed that many research trials initiated by pharmaceutical companies often modified WHO criteria to address areas that lacked clarity and also to accommodate new technologies (such as multidetector CT [MDCT]), and this led to variability and potential for overestimation or underestimation in clinical trial results.
Response Evaluation Criteria in Solid Tumors Criteria
RECIST 1.0
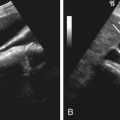
To address limitations of the WHO criteria, an International Working Committee was formed to standardize WHO criteria so that meaningful comparisons could be made among clinical trial studies. This led to the proposed RECIST criteria (version 1.0) in 2000. Important updates for RECIST criteria include adoption of unidimensional measurement (i.e., longest diameter) in RECIST versus bidimensional measurement in the WHO ( Figure 85-1 ), specification on tumor type that should be selected as tumor target, number of tumor targets in total and per organ to be chosen, broadening of the cutoff point defining progressive disease, and recommending the type of imaging that should be used. Even with introduction of RECIST 1.0, some important questions and issues remained unaddressed, such as assessment of lymph nodes, role of new imaging modalities such as PET/CT and MRI and if fewer than 10 lesions can be assessed without affecting the conclusion on response assessment. The RECIST Working Group subsequently revised the original criteria, and RECIST version 1.1 was released in 2008 ( Table 85-1 ).

| Key Features | RECIST 1.1 | RECIST 1.0 |
|---|---|---|
| Measurement method | Longest tumor diameter for extranodal lesions | Longest tumor diameter |
| Target lesion (measurable lesion) size | At least 10 mm on CT | 10 mm on spiral and 20 mm on conventional CT |
| Number of target lesions | Total of 5 and up to 2 lesions per organ | Total of 10 target lesions and up to 5 per organ |
| Assessment of lymph nodes | Short axis measurement recorded. Target nodal lesion ≥15 mm, ≥10 mm, and ≤15 mm pathologic | Not specified |
| Clarification on disease progression | >20% increase in the sum of longest dimensions of target lesions (>5 mm absolute increase is required) and new lesions | >20% increase in the sum of target lesions (no absolute increase is required) and new lesions |
| Imaging modalities | Chest x-ray, CT, and MRI | CT, MRI, and FDG-PET (PET included for lesion detection only.) Chest x-ray can be used but not preferred |
RECIST 1.1
The guidelines defining tumor measurements by RECIST are simple, easy to apply, and quantitative. Optimization of image acquisition protocols at baseline and throughout follow-up examinations is essential for proper application of RECIST 1.1. Contrast-enhanced CT continues to be the most widely used modality. It is recommended to acquire CT images in the portal venous phase of contrast enhancement. For purposes of consistency, it is prudent to reconstruct 5-mm slices (or less) contiguously. To avoid partial volume averaging effects and inconsistent measurements of the same lesion (either target lesion or lymph node or non–lymph node) between serial examinations, the target lesion should be at least twice the slice thickness at baseline—that is, 10 mm if slice thickness is 5 mm. This principle is also applicable to MRI examinations. Measurements and comparisons should be performed on the same pulse sequence on serial studies. Measurement on a chest radiograph is acceptable if the lesion is surrounded by pulmonary parenchyma, but is not preferred owing to the lack of 3D.
Measurability of Tumor Burden
Target Lesion (Measurable Lesion)
Extranodal target lesions should meet the size criterion of 10 mm or greater in the longest dimension (see Figure 85-1 ). A lymph node is considered pathologically enlarged and measurable by RECIST 1.1 if the short axis measures 15 mm or greater on contrast-enhanced CT. At baseline and on subsequent posttreatment time points, the longest dimension of extranodal target lesions and short axis measurement of the node is used for assessing tumor burden and to monitor response. With respect to bone lesions, either lytic or mixed lytic-blastic bone lesions with soft tissue components can be considered measurable lesions as long as the soft tissue component meets the definition of measurability. In RECIST 1.1, blastic bone lesions are considered nonmeasurable. When both cystic and solid metastases are present, solid lesions are preferred as selectable target lesions. Lesions located in a previously irradiated area are not considered target lesions unless there is demonstrable progression in lesion size.
Nonmeasurable Lesion
This includes organ lesions with longest diameter less than 10 mm and lymph nodes 10 mm or greater to less than 15 mm short axis. Other truly nonmeasurable lesions include leptomeningeal disease, ascites, pleural or pericardial effusion, lung lymphangitis, and skin carcinomatosis. Lymph nodes with short axis less than 10 mm are considered nonpathologic and should not be recorded or followed.
Tumor Response Evaluation
Measurable disease is defined by the presence of at least one target (measurable) lesion. At baseline, when more than one target lesion is present, all lesions up to a maximum of five lesions in total and a maximum of two lesions per organ representative of all involved organs should be identified as target lesions to record their measurement at baseline and each time point. It is preferable that the target lesion be clearly defined, be suitable for repeat measurement, and not have been previously treated with local-regional therapy. All other lesions and disease sites, including pathologic lymph nodes (as described earlier), are designated as nontarget lesions, and only their presence should be recorded at baseline. A sum of the longest diameters (SLD) for all target lesions is calculated, reported, and followed to assess response. When lesions are too small to measure, a default value of 5 mm is assigned. If they disappear completely, the measurement is recorded as 0 mm. When a target lesion fragments into multiple smaller lesions, the longest dimension of all fragmented portions are added to the sum. If target lesions coalesce, the longest dimension of the resulting coalescent lesion should be taken.
Measurements are not required for nontarget lesions, which are followed as “present,” “absent,” or, uncommonly, “unequivocal progression.” The appearance of unequivocal new lesions indicates disease progression. If there are unequivocal lesions, progression should be reassessed on follow-up examinations.
Response Categories
Target Lesion Evaluation
The defined response on imaging is categorized as CR (extranodal target lesions have disappeared, and all lymph nodes measure <10 mm in short axis), PR (at least a 30% decrease in SLD of tumor burden), PD (tumor burden increase of at least 20% with an absolute change in SLD of at least 5 mm), and SD (neither PR nor PD) ( Table 85-2 ).
| Complete response | Disappearance of all target lesions or lymph nodes decreased to <10 mm in the short axis |
| Partial response | >30% decrease in sum of longest diameters of target lesions |
| Progressive disease | >20% increase in sum of longest diameters of target lesions with an absolute increase of ≥5 mm; new lesions |
| Stable disease | None of the above |
Nontarget Lesion Evaluation
Any nontarget lesions should be assessed only qualitatively at all time points even if they have measurable size. The responses are categorized as CR (disappearance of all nontarget lesions, normalization of tumor marker level and regression of pathologic lymph nodes to <10 mm in short axis), Non-CR/non-PD (persistence of one or more nontarget lesion(s) and/or maintenance of tumor marker level above the normal limits), and PD (unequivocal progression of existing nontarget lesions) ( Table 85-3 ).
| Target Lesions | Nontarget Lesions | New Lesions | Overall Response |
|---|---|---|---|
| CR | CR | No | CR |
| CR | Non-CR/non-PD | No | PR |
| CR | NE | No | PR |
| PR | Non-PD or NE | No | PR |
| SD | Non-PD or NE | No | SD |
| PD | Any | Yes or No | PD |
| Any | PD | Yes or No | PD |
| Any | Any | Yes | PD |
| Not all evaluate | Non-PD | No | NE |
Overall Response
Based on response outcome of target and nontarget lesion(s) evaluation, overall response is evaluated ( Table 85-3 ).
Although RECIST has gained popularity in evaluating treatment response, it has inherent limitations such as subjectivity in lesion selection and inconsistent imaging protocol. Moreover, with targeted therapies, morphologic changes within tumors lag behind physiologic and molecular changes. Hence, RECIST is not suited to monitor early effects of these novel therapies.
Role of Volumetric and Functional Imaging
Due to lack of standardization, widespread availability, and being labor intensive (in the case of volumetric technique), the working group has not adopted these methods in response assessment by RECIST. The only exception is utility of fluorodeoxyglucose (FDG)-PET to complement CT in assessing disease progression. A positive follow-up FDG-PET with negative FDG-PET at baseline is considered disease progression. In the setting of positive follow-up FDG-PET and absent baseline FDG-PET, new disease site is confirmed where there is a correlate on CT, otherwise follow-up CT scan is recommended to confirm this (if confirmed, the date of abnormal FDG-PET scan will be the date of PD). A positive FDG-PET at follow-up that correlates to anatomically stable disease is not considered PD.
Role of Volumetric and Functional Imaging
Due to lack of standardization, widespread availability, and being labor intensive (in the case of volumetric technique), the working group has not adopted these methods in response assessment by RECIST. The only exception is utility of fluorodeoxyglucose (FDG)-PET to complement CT in assessing disease progression. A positive follow-up FDG-PET with negative FDG-PET at baseline is considered disease progression. In the setting of positive follow-up FDG-PET and absent baseline FDG-PET, new disease site is confirmed where there is a correlate on CT, otherwise follow-up CT scan is recommended to confirm this (if confirmed, the date of abnormal FDG-PET scan will be the date of PD). A positive FDG-PET at follow-up that correlates to anatomically stable disease is not considered PD.
Response Assessment Based on Tumor Density Measurement
Choi Criteria
Tumor x-ray attenuation measured in Hounsfield units (HU) on contrast-enhanced CT can serve as an additional method for response assessment, as described by Choi. Its role was first studied in gastrointestinal stromal tumors (GISTs) treated with imatinib mesylate. Unlike regular chemotherapy, the targeted therapies differ in mechanism of action, such as inhibiting tumor angiogenesis. Tumors treated with targeted therapies may not demonstrate change in tumor burden as seen with standard cytotoxic therapies. Therefore, traditional criteria based on tumor burden assessment cannot be applied to assess response for tumors such as GISTs when treated with targeted therapies.
Reduced tumor radiodensity on portal venous phase CT has shown a correlation with underlying tumor necrosis after therapy without substantial changes in tumor bulk. Tumor attenuation is measured by drawing a region of interest (ROI) circumscribing the margin of the tumor in the portal venous phase. The average Hounsfield units of the target lesions is computed before and after therapy. Response categories and definitions are provided in Table 85-4 . Hounsfield unit measurement has the advantage of being a simple approach, does not require expertise, and can be performed on a routine imaging workstation. The Choi response criteria have been used in assessment of other solid tumors. Some of the studies have suggested utility of Choi criteria in response evaluation of early metastatic renal cell carcinoma treated with sunitinib and in soft tissue sarcoma treated with chemotherapy and radiation therapy.