Objectives
Diagnosis
Evaluate typical morphological and functional characteristics of CMP and differential diagnosis (red flags)
Prognosis
Imaging indices with prognostic value: LV dilation and systolic/diastolic dysfunction (RFP), LV remodeling and dyssynchrony, LV viability, RV involvement, functional MR and TR, intracavitary thrombosis, LA dilation, pulmonary hypertension, LV OT gradient, LGE, abnormal MIBG uptake
Follow-up
Detect changes in functional cardiac parameters
Evaluate reversible forms of CMP
Treatment
Measure LV EF for indication to ICD or CRT
Detect valvulopathy for correction (percutaneous vs. surgical)
Detect ischemia for revascularization
Detect intracavitary thrombosis for anticoagulation
Role in the evaluation for heart transplantation
LV OT obstructive gradient for septal reduction
Indicate pericardial resection in CP
Screening
Familial disease forms
3.2 Diagnosis
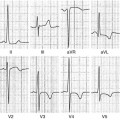
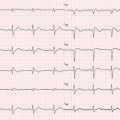
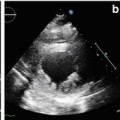
Transthoracic echocardiography (TTE) is an optimal, low-cost, highly reproducible, noninvasive diagnostic tool that can often provide a comprehensive evaluation of the typical morphological and functional characteristics of CMP. However, different CMP can share common echocardiographic features, and differential diagnosis among the specific types of CMP can be particularly difficult. For example, left ventricular (LV) dilation and systolic dysfunction can be found not only in idiopathic dilated cardiomyopathy (DCM) but also in advanced hypertrophic cardiomyopathy (HCM), arrhythmogenic right ventricular cardiomyopathy (ARVC) with biventricular involvement, myocarditis, and – more commonly – in ischemic, hypertensive, or valvular heart diseases [1]. Some authors have therefore suggested the search for diagnostic clues (red flags) to identify specific pathologies and discriminate among the various diseases [2]: namely, in the presence of a hypertrophic phenotype, a ground-glass appearance of the myocardium associated with increased thickness of interatrial septum, atrioventricular valves, and right ventricular (RV) wall, as well as mild pericardial effusion, are imaging clues suggesting the diagnosis of cardiac amyloidosis (CA). Furthermore, transesophageal echocardiography (TEE) and stress echocardiography can add some information in specific patients. In particular, TEE can better assess the mechanism and severity of valvular diseases; dobutamine stress echocardiography can detect a biphasic response typical of ischemic heart disease and distinguish between true aortic valve stenosis and pseudostenosis associated with DCM.
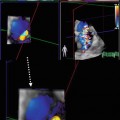
Advanced noninvasive imaging techniques, such as cardiac magnetic resonance (CMR) imaging, computed tomography (CT), and nuclear imaging, can be useful in selected and more challenging cases in which echocardiography does not provide a definite diagnosis. CMR, in particular, permits a highly accurate measure of LV and RV volumes, ejection fraction (EF), wall thickness, and mass; moreover, it is considered the diagnostic method of choice for anatomical and functional RV evaluation in suspected ARVC [3] and for distinguishing between LV noncompaction versus DCM with prominent apical trabeculations or apical HCM [4]. The presence and distribution pattern of late gadolinium enhancement (LGE) is paramount in differentiating ischemic heart disease from other forms of CMP [5]. In case of suspected myocarditis, CMR can provide additional information, detecting myocardial edema in the acute phase of the disease [6]. Moreover, thanks to its high negative predictive value for coronary artery disease diagnosis, coronary angio-CT is useful to distinguish between ischemic and nonischemic CMP [7]. Nuclear imaging can add important information regarding myocardial perfusion and viability. Furthermore, sympathetic cardiac imaging is now employed to study myocardial intake of 123-metaiodobenzylguanidine (MIBG), which is significantly reduced in idiopathic DCM [8].
3.3 Prognosis
The major prognostic echocardiographic markers in DCM patients are the amount of LV dilation and systolic dysfunction, LV remodeling and dyssynchrony [9], RV involvement [10], functional mitral regurgitation (MR) [11], intracavitary thrombosis [12], and a restrictive filling pattern (RFP) of the LV [13]. A previous study demonstrated the value of monitoring the LV filling pattern with TTE during invasive treatment: reversibility of the RFP was correlated with better long-term outcome [14]. Also, reassessment of the LV filling pattern after optimal medical therapy is essential, given the fact that a persistent RFP denotes a worse prognosis [15]. Finally, the presence of viability shown on a dobutamine stress test is correlated with better survival and higher likelihood of improved LV function in DCM patients [16].
Regarding HCM, several echocardiographic parameters have demonstrated prognostic value: a maximum wall thickness >30 mm is considered one of the major risk factors for sudden death (SD); moreover, a resting LV outflow tract (OT) gradient >30 mmHg, presence of LV systolic dysfunction, apical aneurysms, increased LV mass, and left atrial (LA) dilation denote a worse prognosis [17–19]. As in DCM, evidence of diastolic dysfunction has clinical and prognostic value also in HCM: the presence or persistence of RFP identifies more symptomatic patients with a significantly worse prognosis and with increased risk of evolution to end-stage HCM [17, 20].
In restrictive cardiomyopathy (RCM), transmitral- and pulmonary-vein-flow Doppler imaging patterns not only allow assessment of hemodynamic severity of the disease but also have prognostic value [13]. Furthermore, LA diameter >60 mm is a negative prognostic factor, especially in older patients with advanced New York Heart Association (NYHA) class [21]. In ARVC, evidence of severe RV dilation and dysfunction, as well as LV involvement, portends a worse prognosis [22, 23]. In CA, LV wall thickness ≥15 mm, LV fractional shortening ≤20 %, and presence of RFP characterize patients with lower survival potential [24, 25].
Studies on myocarditis show that LV dysfunction at diagnosis indicates a worse outcome even in the absence of clinical signs of heart failure (HF) [26, 27]. Other authors demonstrated that RV systolic dysfunction is an independent predictor of adverse outcome in these patients [28].
Also CMR provides important information when risk stratifying patients with CMP, particularly by detecting LGE, which is consistent with fibrosis. In DCM patients, baseline presence and transmurality of LGE in a myocardial segment are inversely related to its functional improvement at follow-up, even in optimal medical therapy [29], and are markers of diastolic dysfunction [30]. Presence and extent of LGE also demonstrate an association with adverse outcome [31]. Therefore, according to some authors, LGE might help in the arrhythmic risk stratification of patients, indicating use of an implantable cardioverter defibrillator (ICD) [31]. LGE, particularly in the lateral and posterior LV wall, is also inversely related to functional improvement after cardiac resynchronization therapy (CRT) [32].
In HCM, the evidence of myocardial ischemia (in the absence of epicardial disease) and abnormal myocardial blood flow reserve (compatible with microvascular dysfunction) detected with CMR and positron emission tomography (PET) is associated with arrhythmias, LV remodeling, systolic dysfunction, and risk of cardiovascular mortality [33]. Moreover, presence of diffuse LGE at CMR is a risk factor for SD in HCM, in association with maximal LV wall thickness and evidence of LV OT obstruction [34]. The presence of LGE denotes an increased mortality risk also in patients with myocarditis [35, 36]. Finally, analysis of sympathetic cardiac innervation with MIBG may be prognostically useful in HF patients and may help in selecting patients for ICD therapy: preliminary data indicate that abnormal MIBG uptake is a predictor for ventricular arrhythmia recurrence [37], increased incidence of SD, and appropriate ICD discharges [38]. Also, it is independently related to HF progression and cardiac mortality risk [39].
3.4 Follow-up
TTE is the imaging approach of choice for the follow-up of patients with CMP. Serial TTE evaluations during disease course demonstrate incremental prognostic value compared with the single basal TTE assessment [26, 27]. Furthermore, medical treatment must be adjusted according to clinical parameters as well as to echocardiographic data at follow-up [40]. Additionally, TTE examinations are pivotal for detecting deterioration as well as improvement in functional cardiac parameters, considering the fact that initial indications for aggressive and invasive treatments might no longer persist after optimal medical therapy [41].
In DCM, detecting LV systolic function improvement [42], reverse LV remodeling, and RFP reversibility [15] allows identification of patients with excellent long-term outcome [41].
Furthermore, a subgroup of HCM patients (~5 %) can present evolution with LV remodeling and progressive LV dilation and dysfunction, with wall thinning and diffuse LV hypokinesis resembling DCM. In this phase, a differential diagnosis between the two CMP is particularly difficult; serial imaging evaluation is therefore important to detect disease evolution [43].
Finally, TTE assessment at follow-up is important in assessing the evolution of the reversible forms of CMP (inflammatory, alcoholic, tachy-induced, stress-induced, chemotherapy-induced, hypertensive, peripartum): normalization of echocardiographic parameters once the etiological factor is removed denotes an excellent prognosis [26, 27].
3.5 Treatment
Imaging has an important role in managing patients with CMP and provides useful information necessary for choosing the best treatment option. Accurate imaging assessment of LV EF is important for indicating ICD and CRT. Furthermore, evaluation of mechanical dyssynchrony demonstrates potential value for selecting patients for CRT. Detecting valvulopathy associated with LV dysfunction has important therapeutic consequences: functional MR in DCM can be treated with mitral annuloplasty [44] and percutaneous mitral valve (MV) repair with MitraClip implantation [45]. Echocardiography plays an important role in patient selection, during the procedure, and in the follow-up of these patients. Diagnosing ischemic CMP has potential therapeutic implications such as percutaneous or surgical revascularization, particularly if reversibility of LV dysfunction is demonstrated at dobutamine stress test. Identifying intracavitary thrombosis or spontaneous echocontrast in association with severe LV systolic dysfunction might indicate the need for anticoagulation therapy.
Patients with DCM and severe HF refractory to medical therapy are usually referred to heart transplantation. Indications do not include echocardiographic data. However, a complete echo-Doppler study is helpful in excluding potential contraindications (severe pulmonary hypertension), in selecting alternative treatment strategies, and in supporting prognostic assessment; for instance, persistent RFP might have an additional incremental value in patient selection for this treatment [46].
Detection of a significant LV intraventricular obstructive gradient at rest or during echo stress in HCM patients suggests the possible indication for surgical or percutaneous septal reduction [47]. Contrast echocardiography (with contrast injection in septal coronary vessels during coronary angiography) provides guidance during percutaneous alcohol septal ablation by identifying the vessel that supplies the targeted myocardial territory [48




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree