After onset of ischemic stroke, potentially viable tissue at risk (ischemic penumbra) may be salvageable. Currently, intravenous alteplase is approved for up to 4.5 hours after symptom onset of acute ischemic stroke. Increasing this time window may allow many more patients to be treated. The ability to use MRI to help define the irreversibly damaged brain (infarct core) and the reversible ischemic penumbra shows great promise for stroke treatment. Recent advances in penumbral imaging technology may enable a phase III trial of an intravenous thrombolytic to be performed beyond 4.5 hours using techniques to select patients with penumbral tissue.
Stroke remains a major health care challenge worldwide and is the second leading cause of death and the leading cause of disability in Western countries. Ischemic stroke represents the most common subtype of stroke. An occlusion of a cerebral vessel in acute ischemic stroke may lead to a dramatic reduction of blood flow followed by death of brain cells within minutes. Several acute interventions to improve outcomes after stroke have been introduced recently, and others are still being investigated.
The only acute intervention in ischemic stroke that is proven to be effective is intravenous alteplase (rt-PA) given within first the 4.5 hours after the onset of symptoms. The eligibility of patients undergoing thrombolytic therapy within the current time window is determined using clinical and laboratory criteria and a noncontrast head CT scan (to rule out intracranial hemorrhage). Unfortunately, only approximately 2% to 5% of patients with acute ischemic stroke receive this treatment. Therefore, to increase the number of individuals treated, either more patients must present within the 4.5-hour therapeutic window, or the time window itself must be extended.
An ischemic brain lesion consists of an area of irreversibly injured cells referred to as the ischemic core , intertwined with potentially salvageable ischemic tissue at risk, commonly referred to as an ischemic penumbra . The penumbra consists of ischemic tissue that is functionally impaired and at risk of infarction but that has the potential to be salvaged through reperfusion or other strategies. Imaging studies have shown that salvage of this tissue is consistently linked to clinical recovery and that if not salvaged, it is progressively recruited into the infarct core, thereby worsening the clinical outcome. Several markers of the ischemic penumbra have been studied in animals and humans and several imaging techniques, such as positron emission tomography (PET), Xenon CT (Xe-CT), diffusion-weighted (DWI) and perfusion-weighted (PWI) MRI, and perfusion CT, have been used to estimate penumbral volume. Of these, DWI/PWI and CT perfusion are the most practical and commonly used in selecting patients with acute stroke for reperfusion therapies.
Multimodal MRI offers several important advantages over CT, particularly in the extent of information provided that pertains to the diagnosis, management, and prognosis of an acute stroke. DWI can confirm the diagnosis of acute ischemic stroke within minutes after the onset and can estimate the volume and location of the infarct core. PWI assesses cerebral hemodynamics. The mismatch between the infarct core on DWI and the hypoperfusion region on PWI has been hypothesized to represent an imaging surrogate for the ischemic penumbra. In addition, MR angiography (MRA) provides information on major vessel patency, and gradient echo (GRE) and fluid-attenuated inversion recovery (FLAIR) imaging detect intracranial hemorrhage, as does CT. All of these attractive features have inspired several clinical trials to investigate the ability of MRI to improve the selection of patients most likely to respond to treatment in delayed time windows. This article discusses the discoveries and insights gained from these trials and ongoing investigations to improve the ability of MRI to select patients for reperfusion therapies.
Diffusion MRI
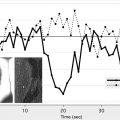
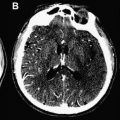
In a normal state random diffusion of water molecules occurs across cell membranes. Tissue damage and ischemia begin to occur at a cerebral blood flow value of approximately 30 mL per 100 g per minute. Severe ischemia of brain tissue induces the rapid dysfunction of cellular metabolism and ion exchange pumps responsible for adenosine triphosphate (ATP) metabolism. This dysfunction causes a massive shift of water from the extracellular to the intracellular compartment, leading to cytotoxic edema that is associated with restricted diffusion of water molecules. This reduction in water diffusion can be quantified by a decrease in the apparent diffusion coefficient (ADC) and can be visualized as an increased signal on DWI. In contrast to CT, in which brain ischemia is typically not visualized for several hours, diffusion signal increases within minutes of stroke onset. Several studies have confirmed the greater sensitivity of DWI for detecting stroke compared with CT scanning or conventional MRI during the acute period ( Fig. 1 ). Thus, in the evaluation of acute stroke, DWI increases diagnostic confidence and may help identify candidates for reperfusion therapies.
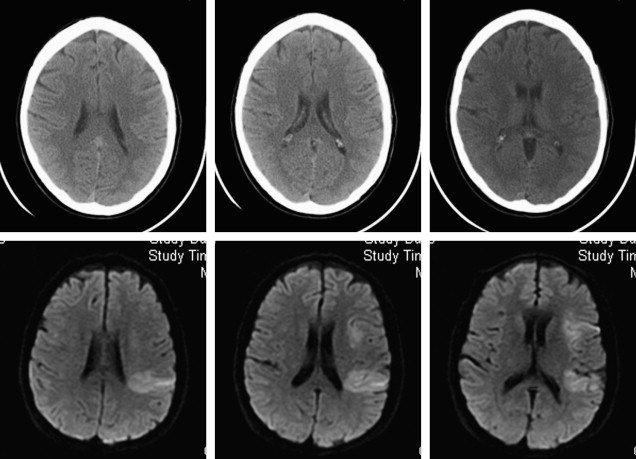
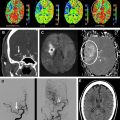
DWI may also yield important prognostic information in the evaluation of acute stroke. Large-volume hemispheric ischemic strokes that cause severe space-occupying edema with the risk of resultant brain herniation are called malignant infarctions ( Fig. 2 ). Early DWI lesion volumes larger than 145 mL predict malignant infarction. Despite medical treatments to reduce intracranial pressure, their prognosis is poor, with fatality rates of nearly 80%. A life-saving emergent surgical decompression (hemicraniectomy), if performed early, reduces mortality and increases the chance for favorable functional outcome in patients with malignant infarction. DWI lesion volume larger than 145 mL is now a validated criterion for emergent hemicraniectomy.
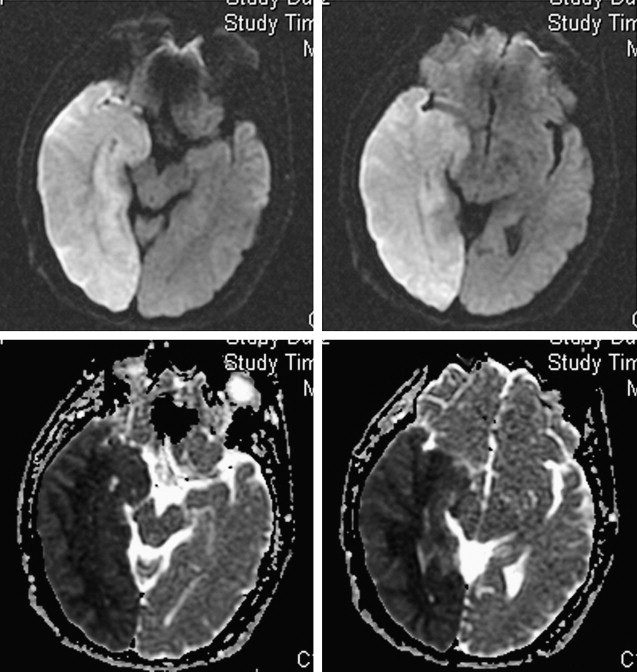
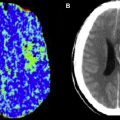
In addition to providing information regarding which patients may benefit from emergent surgical decompression, DWI lesions may also predict the risk of hemorrhagic transformation after thrombolysis. The risk for symptomatic intracerebral hemorrhage after thrombolysis has been associated with pretreatment DWI lesion size. Additionally, areas of severe ADC reduction have been reported to predict subsequent hemorrhagic transformation. In the Diffusion and Perfusion Imaging Evaluation For Understanding Stroke Evolution (DEFUSE) study, patients with a “malignant MRI profile” characterized by a DWI lesion larger than 100 mL and/or a PWI lesion larger than 100 mL with longer than 8 seconds of T max delay had a high risk of symptomatic hemorrhagic transformation and death after rt-PA therapy. Furthermore, in a more recent study, patients with a DWI lesion larger than 70 mL before intra-arterial thrombolysis had a poor prognosis and high mortality rate despite a 50% recanalization rate. The large volume of severe ischemic brain and microvascular injury in these malignant infarcts may increase the risk for reperfusion-related hemorrhage. Excluding patients with unfavorable imaging profiles from recanalization therapies may improve the safety and efficacy of these therapies.
The degree of reduction in ADC values may also predict DWI lesion reversal after recanalization/reperfusion. Several studies have shown that, for patients who do not have a malignant stroke profile, acute DWI lesions can be partially reversed through recanalization/reperfusion. These findings indicate that an acute DWI lesion does not necessarily represent irreversible injury, and may contain a portion of the ischemic penumbra. ADC values typically decrease sharply soon after stroke onset. In animal studies, the reduction in ADC in acute stroke has been shown to occur before irreversible failure of energy metabolism. Other supportive data have emerged from voxel-based comparisons between MRI and PET. Portions of acute DWI lesions show PET scan characteristics of penumbra. Higher ADC values and successful early reperfusion are factors associated with DWI reversal. In the DEFUSE study, an ADC threshold of 615 × 10 −6 mm 2 /s or less provided a sensitivity of 80% and specificity of 77% for identifying the infarct core. Thus, ADC thresholds may be useful to help distinguish ischemic core from penumbra.
Perfusion MRI
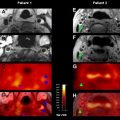
Bolus tracking perfusion imaging using a paramagnetic contrast agent (eg, gadolinium) is the most commonly used MRI technique for assessing cerebral perfusion. The delay in gadolinium arrival to the vascular bed and the intensity of the signal change induced by its passage within capillaries can be used to generate a time/concentration curve. Various maps and parameters can be generated from these data, including cerebral blood flow (CBF), cerebral blood volume (CBV), and time-based factors such as T max , mean transit time (MTT), and time to peak (TTP) ( Fig. 3 ).
The concept of the perfusion-diffusion (PWI/DWI) mismatch for selecting patients for thrombolysis was introduced with several small case series approximately 10 years ago, followed by larger series by several international groups over the past few years. Studies have used multiple different perfusion parameters and thresholds to define critical hypoperfusion as the PWI lesion that will predict the extent of final infarction if the tissue does not experience early reperfusion. T max , the time to peak of the residue function, indicating a delay in bolus arrival between the site of selection of the arterial input function and the tissue, was used to define critical hypoperfusion in both the DEFUSE trial and the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET). These studies selected a T max value of greater than 2 seconds to threshold the PWI lesions in an attempt to exclude benign oligemia. However, retrospective analyses of these studies showed that higher T max thresholds provide a more accurate assessment of critical hypoperfusion. For instance, a post hoc analysis of DEFUSE showed that a PWI lesion with a T max delay between 4 and 6 seconds provides a better estimation of final infarct volume in patients who did not reperfuse compared with the threshold of greater than 2 second threshold. These findings are supported by the results of direct voxel-to-voxel comparisons between MRI PWI and either PET or Xe-CT scans that suggest that 4- to 6-second T max delays correspond most closely with penumbral cerebral blood flow values on PET or Xe-CT. A pooled analysis of both DEFUSE and EPITHET datasets presented at the International Stroke Conference in February 2010 showed that PWI lesions larger than 125 mL defined by a T max threshold of greater than 8 seconds were associated with significantly worse clinical outcomes in patients treated with rt-PA versus placebo.
Overall, the refinement of PWI techniques for acute stroke is ongoing. PWI does not provide an accurate quantitative estimate of cerebral blood flow in individual stroke patients compared with Xe-CT or PET gold standards. Corrections for contrast delay and dispersion seem to improve PWI performance. Although penumbral imaging algorithms are part of several guidelines, including those of the American Heart Association and European Stroke Organization, the ability of DWI/PWI mismatch to detect penumbra requires further validation using quantitative methods.
Perfusion MRI
Bolus tracking perfusion imaging using a paramagnetic contrast agent (eg, gadolinium) is the most commonly used MRI technique for assessing cerebral perfusion. The delay in gadolinium arrival to the vascular bed and the intensity of the signal change induced by its passage within capillaries can be used to generate a time/concentration curve. Various maps and parameters can be generated from these data, including cerebral blood flow (CBF), cerebral blood volume (CBV), and time-based factors such as T max , mean transit time (MTT), and time to peak (TTP) ( Fig. 3 ).
The concept of the perfusion-diffusion (PWI/DWI) mismatch for selecting patients for thrombolysis was introduced with several small case series approximately 10 years ago, followed by larger series by several international groups over the past few years. Studies have used multiple different perfusion parameters and thresholds to define critical hypoperfusion as the PWI lesion that will predict the extent of final infarction if the tissue does not experience early reperfusion. T max , the time to peak of the residue function, indicating a delay in bolus arrival between the site of selection of the arterial input function and the tissue, was used to define critical hypoperfusion in both the DEFUSE trial and the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET). These studies selected a T max value of greater than 2 seconds to threshold the PWI lesions in an attempt to exclude benign oligemia. However, retrospective analyses of these studies showed that higher T max thresholds provide a more accurate assessment of critical hypoperfusion. For instance, a post hoc analysis of DEFUSE showed that a PWI lesion with a T max delay between 4 and 6 seconds provides a better estimation of final infarct volume in patients who did not reperfuse compared with the threshold of greater than 2 second threshold. These findings are supported by the results of direct voxel-to-voxel comparisons between MRI PWI and either PET or Xe-CT scans that suggest that 4- to 6-second T max delays correspond most closely with penumbral cerebral blood flow values on PET or Xe-CT. A pooled analysis of both DEFUSE and EPITHET datasets presented at the International Stroke Conference in February 2010 showed that PWI lesions larger than 125 mL defined by a T max threshold of greater than 8 seconds were associated with significantly worse clinical outcomes in patients treated with rt-PA versus placebo.
Overall, the refinement of PWI techniques for acute stroke is ongoing. PWI does not provide an accurate quantitative estimate of cerebral blood flow in individual stroke patients compared with Xe-CT or PET gold standards. Corrections for contrast delay and dispersion seem to improve PWI performance. Although penumbral imaging algorithms are part of several guidelines, including those of the American Heart Association and European Stroke Organization, the ability of DWI/PWI mismatch to detect penumbra requires further validation using quantitative methods.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree