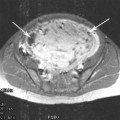
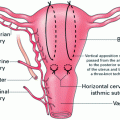
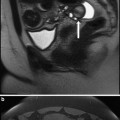
Fig. 1
Diagram demonstrating the classification of abnormal placentation dependant on the depth of penetration into the myometrium
2.1.3 Placenta Previa Accreta
The two above conditions may co exist where the placenta is inserted low and is morbidly adherent.
2.2 Incidence
The incidence of abnormal placentation is on the rise and is associated with the increased caesarean section rate (Clark 1985, 1986; To 1995; Wu 2005; Usta 2005; Silver 2006; Solheim 2011). In addition, the chance that the placenta is not only previa but also accreta also increases with the number of caesarean sections. It follows that percentage of women requiring hysterectomy as a result of this condition will also increase. A study from the US following a cohort of over 30,000 women who delivered by caesarean section demonstrated placenta accreta to complicate 0.24 % of first pregnancies, 0.42 % of second, and rising incrementally to reach an incidence of 8.99 % for sixth or more caesarean section deliveries. In the cohort with placenta previa, the risk of placenta also being accreta was 3, 11, 40, 61 and 67 % for first, second, third, fourth and fifth or more caesarean sections (Silver 2006). Forecasts from the US where caesarean section rates reached 32.9 % in 2009 predicts that the rate of caesarean sections will rise to 56.2 % by 2020 and will be associated with many more previas, accretas and likely maternal deaths (Solheim 2011).
2.3 Diagnosis
2.3.1 Ultrasound
Vaginal bleeding after 20 weeks should raise the suspicion of placenta previa. Routine transabdominal US at 20 weeks will also allow for identification of previa. If detected, then transvaginal imaging may be indicated to further investigate/reclassify the diagnosis (Smith 1997; Lauria 1996). In high risk patients with a major previa or accreta at the 20 week scan repeat imaging with US is recommenced at 32 weeks to clarify the diagnosis and plan multidisciplinary strategies for delivery (Royal College of Obstetricians and Gynaecologists 2011).
2.3.2 MRI
Two recent studies comparing the sensitivity and specificity of US versus non-contrast MRI showed no difference in their abilities to detect placenta accreta (Dwyer 2008; Masselli 2008) though one of these studies demonstrated that MRI was better at demonstrating the depth of penetration of the placenta (Masselli 2008). Thus, MRI may provide a useful tool for problem solving in difficult cases.
3 Preparations for Delivery in Placenta Previa/Previa Accreta
The most recent RCOG guidelines concerning the diagnosis and management of placenta previa/previa accreta recommend that “all women with placenta previa and their partners should have a discussion regarding delivery, indications for blood transfusion and hysterectomy” recognising the serious nature of the condition and potential devastating complications. The mode of delivery is based on the clinical judgment and US findings but any woman with a placenta less than 2 cm from the cervical os is likely to require caesarean section. Elective caesarean section is recommended with uncomplicated placenta previa planned for 38–39 weeks and for women with placenta accreta at 36–37 weeks (Royal College of Obstetricians and Gynaecologists 2011).
In the UK, a six point care bundle has been developed by the National Patient Safety Agency, Royal College of Obstetrics and Gynaecology, and Royal College of Midwives for the treatment of placenta accreta (Paterson-Brown and Singh 2010). This involves: 1. a consultant obstetrician supervising the delivery, 2. a consultant anaesthetist supervising the anaesthetic, 3. the availability of blood and blood products 4. multidisciplinary pre-operative planning 5. the local availability of a level 2 critical care beds and 6. discussion and consent concerning possible interventions (including interventional radiology).
3.1 Management of Placenta Previa/Accreta
Placenta previa where uncomplicated is unlikely to require input from interventional radiology as planned caesarean section is less likely to result in massive haemorrhage or emergency hysterectomy. Some major previa cases may require IR input if post partum haemorrhage occurs and internal iliac arterial embolisation is desired. Accurate antenatal scanning of placental position and depth of involvement is essential in forewarning the clinician to the appropriate preparations, but even then not all cases of placenta accreta can be diagnosed antenatally. Placenta previa and a history of caesarean sections should lead to a high index of suspicion.
Placenta accreta is associated with a higher incidence of complications morbidity and mortality and various strategies for conservative and operative management have been recommended. The abnormal penetration of the placenta complicates placental detachment at caesarean section and in all but those patients with focal accreta then hysterectomy without attempting to separate the placenta is recommended (Oyelese 2006). Conservative management in the form of leaving the placenta in situ has been attempted in selected cases but may be associated with complications such as infection, delayed haemorrhage and reoperation requiring hysterectomy (Timmermans 2007). Even where hysterectomy is planned control of haemorrhage may be challenging and expert Obstetric surgery is mandated. It is in these cases that transcatheter techniques may have an important role to play in prophylactic and emergency control of haemorrhage. Placenta percreta involving the bladder is of particular concern as torrential haemorrhage, bladder injury and urethral damage are not uncommon when attempting hysterectomy (Abbas 2000).
3.2 Interventional Radiology and Transcatheter Techniques in the Management of Abnormal Placentation
The routine 20 and 32 week US scanning (with the aid of MRI in some selective cases) will select the deliveries that may require the support of Interventional Radiology.
Intra arterial balloon occlusion for control of bleeding was described as far back as the Korean war (Edwards 1953; Hughes 1954) and transcatheter embolisation techniques in the treatment of obstetric and gynaecological emergencies have been reported since 1979 (Brown 1979).
Transcatheter techniques in the context of abnormal placentation have included:
Prophylactic internal iliac occlusion balloon catheters (IIOBC).
Prophylactic (internal iliac) arterial catheterisation, balloon occlusion and embolisation (PACBOE).
Emergency transcatheter arterial embolisation.
Occlusive balloon catheters in other sites e.g., aortic or common iliac.
3.3 Evidence for Transcatheter Techniques in Abnormal Placentation
There are no randomised controlled trials to help support the use of transcatheter techniques in abnormal placentation. The published literature concerning transcatheter techniques in abnormal placentation consists of uncontrolled case reports and a few small case-controlled studies.
3.3.1 Prophylactic Internal Iliac Occlusion Balloon Catheters
In one of the earliest series in the literature, (Levene 1999) followed five patients with placental accreta prospectively who delivered with the aid of prophylactic balloon occlusion and compared them to a control group numbering four patients. Balloon positioning was inconsistent amongst this study group (internal iliac n = 7, anterior division n = 1 and uterine n = 2). This study demonstrated no significant reduction in estimated blood loss (mean 5,025 ml vs. 4,653 ml), transfusion requirements (5.5 units vs. 4 units) or hospital stay (7 days vs. 5 days). Given the small size of the study it is difficult to extrapolate much from the conclusion that no significant differences were found in their outcome measures.
The largest single retrospective case series study (Shrivastava 2007) looked at blood loss, transfusion requirements, operative time and hospital stay for 69 women who underwent hysterectomy for placenta accreta. Of these women, 19 had internal iliac artery balloon occlusion at delivery. No difference in the primary outcomes was demonstrated. The balloon occlusion group demonstrated significant complications in three patients who were directly related to balloon placement. Two patients required bypass surgery and thus the authors concluded that it was difficult to support the use to IIOBCs where there was no perceived benefit and significant attendant risks to their placement.
There is also support for the use of IIOBCs in the literature, one of the earliest being a retrospective study of five women with potential accreta who underwent caesarean section and hysterectomy with the aid of prophylactic IIA balloon occlusion catheters (Kidney 2001). Placenta accreta was present in three patients; the remaining two patients having previa alone. Blood loss was estimated at between 1,100 and 4,000 ml and two patients required no blood products implying the usefulness of the technique though no control group was included for comparison.
More recently, a retrospective case-controlled study reviewed the cases of 25 women with placenta accreta who underwent elective caesarean section (Tan 2007). Eleven women had preoperative balloon occlusion compared to a 14 patient control group. This demonstrated a significantly lower intra operative blood loss, transfusion requirement and operative caesarean section time in the balloon occlusion group. No significant complications were recorded with respect to the placement of the balloon catheters and vascular access in this series.
Another retrospective case series is also supportive (Carnavale 2011), they looked at 21 patients with placenta accreta diagnosed antenatally by US and MRI. All had inflation of IIOBC at the time of cord clamping and CS followed by hysterectomy. Estimated blood loss was between 200 and 4,000 ml, a blood loss similar to other studies utilising the same IIOBC techniques. However, they also reported two patients requiring surgical thrombectomy for lower limb embolic complications related to balloon placement.
The importance of accurate prenatal diagnosis cannot be overstressed as demonstrated in a retrospective study of 2008 (Mok 2008). In this study of 13 patients (12 of which had prophylactic IIA occlusion balloons placed prior to caesarean section for suspected previa accrete) blood loss varied between 300 and 14,000 ml. Of note, six women did not require balloon inflation as previa only was encountered at operation and two patients had balloons inflated though previa only was encountered. The sensitivity and specificity of the prenatal diagnosis was poor in this study at 38 and 33 %, respectively. It may be argued that this may expose some women unnecessarily to the risk of complications from the placement of IIOBCs.
Another retrospective case series (Thon 2011) assessed the success of prophylactic balloon occlusion in suspected placenta previa accreta by chart review and retrospective questionnaire. Of the 14 women, 7 were found to have previa alone at CS. Uncomplicated separation of the placenta occurred in three of the seven placenta previa group, thus the IIOBCs were not necessary. The remaining 11 patients had IIOBCs inflated, in six cases it was deemed efficacious, deemed unnecessary in one further case and did not help in other four instances. Blood loss in this group varied between 700 and 15,000 ml and nine patients required hysterectomy. This case series recorded a number of complications, including groin haematoma, IIOBC migration and lower limb arterial insufficiency.
There are also a number of single case reports supportive of prophylactic IIOBC (Weeks 2000; Shih 2005; Yi et al. 2010) all describing balloon occlusion without embolisation in placenta accreta. Low blood losses 800–1,500 ml were recorded in these reports. Two cases were of the increta type (Weeks and Yi) and one percreta (Shih). Interestingly, the reported success of the placenta percreta case was achieved with the use of common iliac balloon occlusion.
3.3.2 Prophylactic Arterial Catheterisation, Balloon Occlusion and Embolisation
Additional embolisation with gelatine sponge is a further option in the treatment of placenta accreta. The rationale for additional embolisation has been intimated from the apparent failure of surgical IIA ligation observed in some surgical series (Clarke et al. 1985; Evans and Mcshane 1985) due to extensive pelvic collateralisation.
One of the earliest reports in the literature (Dubios 1997) concerned two cases of placenta percreta. The axillary arteries were used for access and balloon occlusion was followed with gelatine sponge embolisation. They described good outcomes with blood loss between 1,500 and 2,000 ml.
A case-controlled series (Bodner 2006) of 28 patients with placenta accreta included six patients who underwent IIOBC insertion, caesarean section and transcatheter gelatine foam embolisation prior to either hysterectomy (n = 5) or uterine curettage (n = 1). This group was compared to 22 patients who underwent caesarean hysterectomy alone. The authors admit that there were significant differences in pre-delivery hospitalisation in the embolisation group and that the embolisation group were also delivered at an earlier gestational age. The two groups also differed in size thus it is difficult to extrapolate much from the findings that there was no difference in estimated blood loss, volume of replaced blood products, fluid replacement needs, operating time or post-operative recovery time.
Another retrospective case series in the literature reviewed 22 patients treated for obstetric haemorrhage of a variety of causes with transcatheter techniques (Ojala 2005). A subset of seven patients had prophylactic IIA catheterisation and arterial embolisation for placenta previa. These seven patients had a prenatal diagnosis of placenta previa (two with concomitant accreta one with percreta). One of the accreta patients and the percreta case required hysterectomy and encountered large blood losses 4,000–10,000 ml despite balloon occlusion and embolisation.
A further small retrospective series of six patients with obstetric haemorrhage (Hansch 1999) contained two patients with placenta previa accreta. In one patient, despite the IIOBC and gelatine foam embolisation, haemostasis could not be obtained and hysterectomy was preformed (with an estimated blood loss of 4,000 ml). The second previa accreta case and IIOBCs was reported as being effective in reducing blood loss at CS. Bleeding, however, resumed 10–15 min later presumably as the result of collaterals and gelatine foam embolisation was performed of the internal iliac arteries, this patient avoided hysterectomy but had an estimated blood loss of 3,300 ml. The other four patients in this study demonstrated a variety of other obstetric haemorrhagic conditions that responded variably to IIOBC and embolisation.
The term prophylactic pelvic artery catheterisation, balloon occlusion and embolisation (PACBOE) was coined in 2010 (Sivan 2010). They retrospectively reviewed 30 cases with suspected placenta accreta, 25 were demonstrated to be placenta accreta at CS. In eight cases, catheterisation was transbrachial and transfemoral in 22. The balloons were inflated at delivery and where bleeding was deemed to be massive then gelatine foam embolisation was additionally performed. The median blood loss in this study was 2,000 ml (500–9,000 ml) but no control group was included for comparison. The transbrachial group had a higher estimated blood loss and operative time compared to the transfemoral group. This report demonstrated a low rate of hysterectomy (8 %) compared to other studies and attributed this to experience acquired in single centre multidisciplinary approach.
PACBOE has been linked to positive outcome in a case report (Faranesh 2007) concerning the treatment the of the feared placenta percreta with bladder involvement. After balloon occlusion and subtotal hysterectomy, the patient was transferred to IR suite for the embolisation (part of the adherent placenta invading the bladder had been left in situ). A four unit transfusion of red cells was required but patient was discharged well at 9 days.
3.3.3 Emergency Transcatheter Embolisation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree