Scrotum and Contents
The most commonly used examination to detect and characterize scrotal and intrascrotal anatomy (Fig. 20.1) and pathology, especially in patients with acute scrotal pain or swelling, is ultrasound (Figs. 20.2 and 20.3), usually with color Doppler imaging. Testicular scintigraphy has been employed in the workup of patients with acute scrotal pain, and is accurate in demonstrating conditions that produce large masses or regions of ischemia or hyperemia, but the higher spatial resolution of ultrasound, and the perfusion information available from Doppler and color Doppler examinations, has led to the more frequent use of ultrasound as an initial examination.
Using surface coils, magnetic resonance imaging (MRI) can produce high-quality images of the scrotum and its contents, but MRI rarely provides important clinical information not obtainable by ultrasound. Computed tomography (CT) is not used to examine the scrotum and its contents, although CT of the lower abdomen may display hernias that extend into the scrotum and undescended testes in the inguinal canals; CT remains the most useful modality to detect abdominal metastases arising from intrascrotal malignancies. CT is also useful to detect and characterize mediastinal and pulmonary metastatic disease.
Scrotal imaging is most commonly used to evaluate patients with scrotal pain, enlargement, palpable masses, or cryptorchidism, but is occasionally employed to work up cases of male infertility. Much of the investigation of such patients involves historical and biochemical data, but scrotal abnormalities may be encountered. Cryptorchidism may be encountered, and prior testicular insults, such as missed torsion, trauma, orchitis (especially mumps), radiotherapy, and steroid abuse, may result in testicular atrophy. Tumors are rare, but more frequent than in the general population, and patients with congenital adrenal hyperplasia may have intratesticular adrenal rests. Obstruction at the level of the epididymis may produce epididymal cysts or enlargement; there may be degrees of epididymal aplasia. Obstruction of the vas or epididymis may cause ectasia of the rete testis. Azoospermia or oligospermia may mandate transrectal ultrasound or MRI of the prostate to search for congenital absence of the vas or seminal vesicle (especially in patients with cystic fibrosis), cysts of the seminal vesicle, and occasional midline cysts such as utricles, mullerian, or wolffian remnant cysts.
▪ INTRATESTICULAR LESIONS
Testicular Tumors
Testicular cancer accounts for approximately 1% of all cancers in men. Testicular tumors may be classified as germ cell tumors, tumors arising from the gonadal stroma, metastases (including lymphoma), and adrenal rest tumors. Germ cell tumors include seminoma, embryonal cell carcinoma, choriocarcinoma, teratoma, and yolk-sac tumors. They may be of single or mixed cell type, and may constitute approximately 95% of testicular tumors and 5% of male genitourinary tumors. Germ cell tumors are the most common solid tumors of men between ages 20 and 34 years. In adults, 40% to 45% of germ cell tumors are seminomas; in order of decreasing frequency, the remainders are embryonal carcinoma, choriocarcinoma, teratoma, teratocarcinoma, and other rare tumors. In children, approximately 70% of testis tumors are of germ cell origin and, of these, yolk-sac tumors and teratomas comprise about 85%.
There is a strong association between testis tumors and cryptorchidism. The risk of a tumor appearing in a cryptorchid testis is more than 30 times that appearing in a normal descended testis, and approximately 10% of testis tumors occur in cryptorchid testes. The histologic distribution of the types of tumors that develop in undescended testes is similar to that of those that develop in scrotal testes. Seminoma is the most common cell type, followed by embryonal carcinoma and teratocarcinoma.
The clinical presentation of testis tumors may involve the detection of an enlarged testis or a scrotal mass by the patient or his physician. The enlargement is often painless; acute scrotal pain may indicate hemorrhage, and suggests diagnoses of torsion or
epididymo-orchitis. Occasionally, an asymptomatic, intratesticular, nonpalpable mass may be encountered in a scrotal ultrasound performed for other reasons; this finding often leads to orchiectomy, whether or not the lesion turns out to be a primary malignancy. In patients with advanced disease, initial symptoms such as supraclavicular lymphadenopathy, dyspnea, and back pain may be caused by metastases.
epididymo-orchitis. Occasionally, an asymptomatic, intratesticular, nonpalpable mass may be encountered in a scrotal ultrasound performed for other reasons; this finding often leads to orchiectomy, whether or not the lesion turns out to be a primary malignancy. In patients with advanced disease, initial symptoms such as supraclavicular lymphadenopathy, dyspnea, and back pain may be caused by metastases.
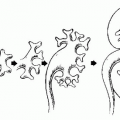
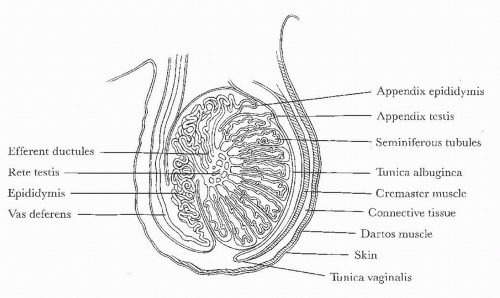 FIGURE 20.1. Diagram of scrotal and testicular anatomy. (From Amis ES Jr, Newhouse JH. Essent Uroradiol. Boston, MA: Little, Brown and Company; 1991, with permission.) |
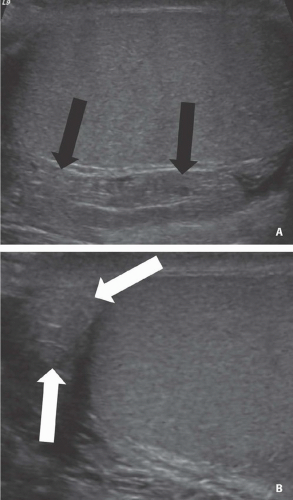
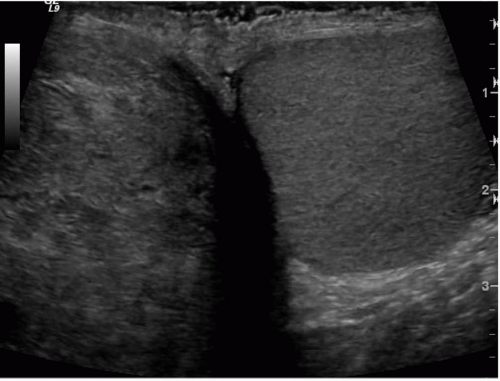
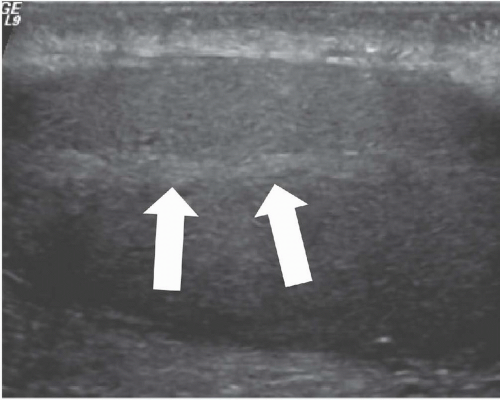 FIGURE 20.2. Mediastinum testis. The band of bright echoes (arrows) represents the mediastinum testis. |
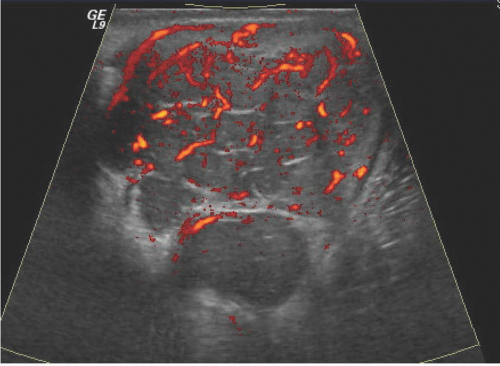
Occasionally, testicular tumors may present with metastases, but without a palpable primary tumor in the scrotum; in these cases, ultrasound is particularly important in searching for the primary tumor. Sometimes the primary tumor is “burned out” and is seen as little more than a hyperechoic area or a linear calcification. Ultrasound of a testis tumor usually reveals the primary lesion to be an intratesticular mass; indeed, deciding whether the mass is in the testis is an important step toward determining whether it is a malignancy because so few extratesticular scrotal lesions are neoplasms. Most testis tumors are shown to be hypervascular by Doppler examination except cystic and very small tumors. The abnormal vessels are commonly disorganized, but may be relatively regular in configuration with infiltrative tumors such as lymphomas and focal leukemic deposits. Intratumoral calcifications may occur, but they are rare.
The sensitivity of scrotal scintigraphy is directly related to tumor size; the masses usually appear as relatively photopenic regions in the location of the testis. There are no reliable scintigraphic characteristics that permit distinction among tumor types. Modalities that directly display testicular parenchymal tissue (ultrasound and MRI) usually reveal a region of normal parenchyma in the involved testis; even if the tumor is quite large, a displaced rim of normal parenchyma can usually be found at some portion of the tumor’s periphery. Ultrasound will show this parenchyma to be similar to the contralateral testis in echogenic pattern; acute or missed torsion, however, will usually cause the entire parenchyma of the involved testis to be abnormal. Orchitis may involve the entire testis or may be focal. When focal, orchitis can usually be distinguished from a tumor by its acute history and tenderness, and by its tendency to appear adjacent to an inflamed epididymis.
Tumors cannot be distinguished accurately on the basis of their sonographic texture; but most seminomas are homogeneous, and most nonseminomatous tumors are not. Small tumors tend to be hypovascular when subjected to color Doppler ultrasound, and larger ones tend to have increased flow; Doppler patterns are not
particularly useful in differentiating tumor types. MRI will almost always reveal solid tumor tissue to be less intense than the normal parenchyma in T2-weighted images; the lesions are often isointense to normal tissue in T1-weighted images. Gadolinium-enhanced MRI is highly accurate in local tumor depiction, and usually reveals details of local stage (breach of the tunica albuginea, invasion of the epididymis, invasion of the scrotal wall, etc.) in depth. Fluorodeoxyglucose-positron emission tomography (FDG-PET) is relatively accurate in initial staging of primary testicular malignancies.
particularly useful in differentiating tumor types. MRI will almost always reveal solid tumor tissue to be less intense than the normal parenchyma in T2-weighted images; the lesions are often isointense to normal tissue in T1-weighted images. Gadolinium-enhanced MRI is highly accurate in local tumor depiction, and usually reveals details of local stage (breach of the tunica albuginea, invasion of the epididymis, invasion of the scrotal wall, etc.) in depth. Fluorodeoxyglucose-positron emission tomography (FDG-PET) is relatively accurate in initial staging of primary testicular malignancies.
Tumors confined to the scrotum are defined as stage I lesions. Stage II disease involves lymph nodes inferior to the diaphragm, stage III disease has metastasized to extra-abdominal lymph nodes, and stage IV connotes extranodal disease. Staging is usually performed by CT of the abdomen and chest. Serologic tumor markers, including α-fetoprotein (AFP) and human chorionic gonadotropin (hCG), are also valuable in assessing initial stage and recurrence, but are often not elevated in patients with pure seminoma.
Testis tumors may spread by direct invasion of the epididymis or spermatic cord, by involvement of retroperitoneal nodes, and by hematogenous dissemination. Retroperitoneal lymph node involvement often appears first at the level of the renal hilum on the left and slightly lower on the right. Inguinal and inferior iliac nodes are usually spared unless the primary tumor has invaded the skin of the scrotum or has recurred in the scrotum after orchiectomy. Continued lymphatic spread may occur through the thoracic duct and to supraclavicular lymph nodes. Hematogenous metastases usually appear in the lungs, where they form multiple pulmonary nodules or masses.
The detection of nodal metastases is based on the size of the lymph nodes, especially in nonseminomatous germ cell tumors (NSGCTs). It is assumed that the larger the lymph node, the more likely it is to contain metastatic disease. Although a measurement of 10 mm in short-axis diameter has often been used as the threshold for the diagnosis of lymph node involvement, this is probably too large, given the image quality of current generation CT scanners. It may be prudent to use a lower threshold for lymph node size in the expected routes of lymph node dissemination and a higher threshold for nodes outside these regions. FDG-PET scans can detect metastases with reasonable accuracy and is increasing in utilization, but to date, it has not replaced anatomic scanning for routine clinical use. Serum tumor markers often aid in detecting and following testicular tumors. Although seminomas only rarely cause elevations in AFP and hCG, nonseminomatous tumors more frequently do, so choriocarcinoma is nearly always accompanied by elevated hCG.
Inguinal orchiectomy involves minimal morbidity and functional impairment and provides excellent local control of testicular neoplasms. Subsequent treatment depends on the stage and histologic nature of the tumor and usually involves combinations of external-beam irradiation, chemotherapy, and retroperitoneal lymphadenectomy. Seminomas are quite sensitive to irradiation and have a cure rate of more than 95% when the diagnosis is made before metastases have occurred; lymphadenectomy is often not needed. NSGCTs are less sensitive to irradiation and usually require a combined therapeutic approach. The 5-year survival rate for patients with stage I disease is 90% to 95%.
Seminoma
Seminomas are the most common testicular neoplasm. They account for approximately 40% of all germ cell tumors. Seminomas occur in slightly older men than do other types of testicular tumors; patients are often 30 to 45 years of age.
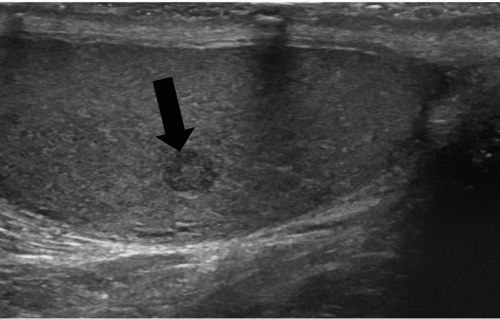
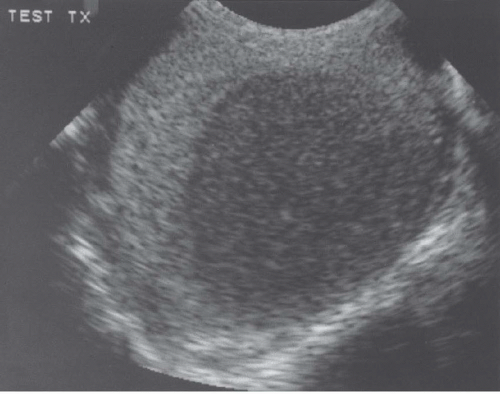
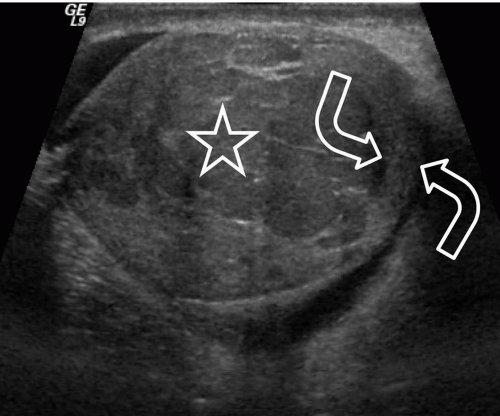 FIGURE 20.5. Seminoma. The tumor (star) in the right testis is heterogeneous; a rim of normal tissue (curved arrows) surrounds it. |
Ultrasound usually reveals testicular seminomas as intratesticular masses that are less echogenic than the adjacent normal testicular tissue (Figs. 20.4, 20.5, 20.6 and 20.7). They may be discrete and small or
may involve most of the testis. Doppler ultrasound usually demonstrates perfusion. Testicular seminomas rarely involve the tunica albuginea, or become necrotic, cystic, or hemorrhagic. T2-weighted MR images usually reveal the lesions to be of lower intensity than the normal testes. The lesions are often uniform in appearance, but may be heterogeneous. Lymph node metastases in the retroperitoneum or mediastinum are manifested as nodal enlargement, which may be detected by either CT or MRI, both of which are roughly equivalent in sensitivity. Malignancy in normal-size nodes is extremely difficult to detect. FDG-PET scans are reliable in evaluating metastases and may be true positive in some cases in which CT is falsely negative.
may involve most of the testis. Doppler ultrasound usually demonstrates perfusion. Testicular seminomas rarely involve the tunica albuginea, or become necrotic, cystic, or hemorrhagic. T2-weighted MR images usually reveal the lesions to be of lower intensity than the normal testes. The lesions are often uniform in appearance, but may be heterogeneous. Lymph node metastases in the retroperitoneum or mediastinum are manifested as nodal enlargement, which may be detected by either CT or MRI, both of which are roughly equivalent in sensitivity. Malignancy in normal-size nodes is extremely difficult to detect. FDG-PET scans are reliable in evaluating metastases and may be true positive in some cases in which CT is falsely negative.
Embryonal Cell Carcinoma
Embryonal cell carcinomas account for approximately 30% of germ cell neoplasms and usually occur in men younger than those who develop seminomas. These aggressive tumors may invade the tunica albuginea and may alter the testicular shape. Sonographically, the lesions are poorly circumscribed, hypoechoic, and more likely to be of nonuniform echogenicity than seminomas (Fig. 20.8). Embryonal cell carcinomas often contain focal areas of increased echogenicity that represent regions of necrosis and hemorrhage and may demonstrate dense echogenic foci. They are easily detected by MRI, but no specific patterns permit reliable differentiation of different types of testicular tumors.
Choriocarcinoma
Choriocarcinoma is less common and accounts for only 2% of testicular tumors. The tumor is aggressive, metastasizes early, and is more likely to present with symptoms from the metastases. The primary lesion may be small; sonography reveals it to be primarily hypoechoic with mixed areas of increased echogenicity due to necrosis, hemorrhage, and calcification.
Lymphoma
Among men 60 years and older, lymphoma is the most common testicular neoplasm. These tumors, which are usually non-Hodgkin lymphomas, constitute approximately 5% of all testicular tumors. In most cases, testicular lymphoma is secondary to disease elsewhere and is not primary in the testis. Lymphoma is the most common bilateral testicular neoplasm.
Testicular lymphoma appears hypoechoic on ultrasound. It has a low signal intensity on both T1- and T2-weighted MR sequences and shows less enhancement than normal testicular tissue. Since testicular lymphoma tends to be aggressive, the diagnosis is more likely if both the testis and the epididymis are involved.
Other Germ Cell Tumors
Most of the remaining germ cell tumors are of mixed histologic pattern and show more than one germ layer. These include teratoma, teratocarcinoma, seminoteratoma, and seminoembryonal cell carcinoma. The most common of these tumors are teratoma and teratocarcinoma. Yolk-sac tumors are rare and occur almost exclusively in young boys. A testicular tumor in a young boy without adrenal abnormality is most likely to be a teratoma or yolk-sac tumor.
Teratomas account for 10% to 20% of testicular tumors. They commonly form well-differentiated squamous cystic lesions containing keratinaceous fatty material, muscle, cartilage, bone, and mucous glandular tissue. Although many of these tumors are considered benign, nearly one-third of patients develop metastases within 5 to 10 years of diagnosis. Ultrasonography shows cystic hypoechoic areas with areas of marked hyperechogenicity.
Teratocarcinoma is a mixture of teratoma and embryonal cell carcinoma, and is slightly less common than seminoma. This tumor is very aggressive, metastasizes early, and may break through the tunica albuginea. It is subject to necrosis and hemorrhage. Ultrasound shows a hypoechoic cystic lesion that is poorly demarcated and may contain areas of increased echogenicity (Fig. 20.9).
Yolk-sac tumors are more likely to metastasize to the lungs than to nodes and frequently produce elevated levels of AFP. Intratesticular papillary adenocarcinoma is a rare cystic malignancy; fewer than 25 cases have been reported. The sonographic findings reported in one case are those of a well-defined, multicystic, septated, intratesticular lesion. Within the cysts are solid echogenic nodules, some of which develop in the septations.
Nongerminal Testis Tumors
Primary tumors that arise from the gonadal stroma are Leydig cell or Sertoli cell tumors. Leydig cell tumors develop in the interstitial cells of the fibrovascular stroma; they may produce testosterone and therefore may result in increased muscle mass. Leydig cell
hyperplasia has been described in the presence of seminoma. Sertoli cell tumors develop from the basement membranes of seminiferous tubules and produce estrogen. These tumors are usually benign and rare. On ultrasound, Sertoli cell tumors cause spotty hypoechoic areas with several associated cysts.
hyperplasia has been described in the presence of seminoma. Sertoli cell tumors develop from the basement membranes of seminiferous tubules and produce estrogen. These tumors are usually benign and rare. On ultrasound, Sertoli cell tumors cause spotty hypoechoic areas with several associated cysts.
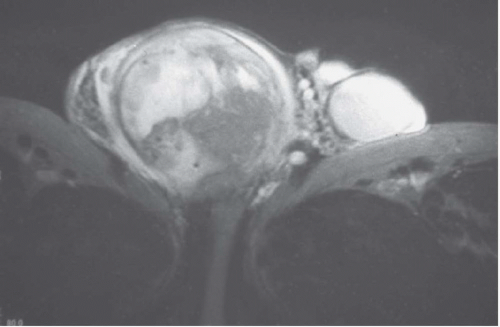
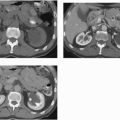
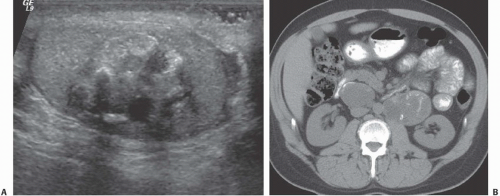 FIGURE 20.9. Teratoma. A: The primary lesion is very heterogeneous. B: CT reveals partially calcified retroperitoneal nodal metastases; in the characteristic location. |
Other rare primary testicular tumors do not arise from germ cells. These include gonadoblastoma, adenocarcinoma of the rete testes, and mesenchymal neoplasms such as fibromas and leiomyomas. Adenomatoid tumors, which are small and benign, may occur in the testis, but are usually found in the epididymis. Metastatic disease to the testis is more common than primary testicular germ cell tumors in men older than 50 years.
Metastases have been reported from a variety of primary tumors arising in the prostate, kidney, lung, gastrointestinal tract, skin, and other sites. Testicular metastases are usually bilateral, multiple, and hypoechoic, although hyperechoic metastases have been observed.
Testicular lymphoma may be primary in the testis without nodal or systemic involvement, or it may be a complication of systemic disease. Lymphoma accounts for 25% of testicular tumors in men older than 50 years. Poorly differentiated lymphoma is more likely to be bilateral. Lymphoma may appear as one or more focal hypoechoic regions (Fig. 20.10) or as a diffusely enlarged hypoechoic testis. Patients with leukemia may develop leukemic deposits in the testes, which look similar to the abnormalities seen with lymphoma (Fig. 20.11).
In patients with leukemia, the testis may be the first site of extramedullary disease and may appear before hematologic relapse. In patients with increased levels of corticotropin (adrenocorticotropic hormone [ACTH]), adrenal rest tumors of the testes may develop. These may occur in patients with congenital adrenal hyperplasia or primary adrenal insufficiency. Testicular adrenal rest tumors appear in most patients with severe congenital steroid deficiency. They probably represent hypertrophy of ectopic adrenal rests that migrated with the testes during fetal development. The tumors are usually multiple and produce testicular enlargement. On ultrasound, the lesions are eccentrically placed intratesticular nodules, which are usually hypoechoic if small and hyperechoic if large; some may produce acoustic shadowing. MRI reveals the tumors to have similar innate characteristics as most other testicular tumors; they are approximately isointense to normal testicular parenchyma on T1-weighted images and hypointense on T2-weighted images.
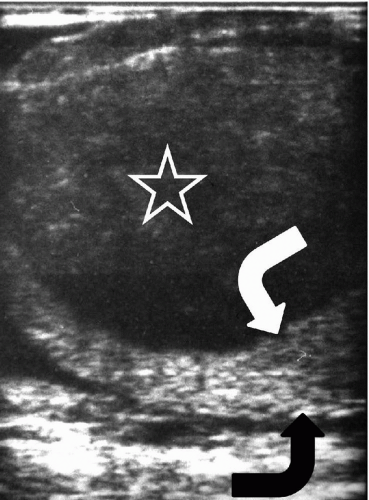 FIGURE 20.10. Lymphoma. A large hypoechoic mass (star) replaces most of the testis; there is a rim of normal tissue remaining (curved arrows). |
▪ TESTICULAR TUMORS
Seminoma
Embryonal cell carcinoma
Choriocarcinoma
Lymphoma
Mixed histology
Nongerminal tumors
Benign Testicular Conditions
Cysts
Testicular cysts are rare. They are usually idiopathic, but may be postinflammatory or posttraumatic. They arise from efferent ductules of the rete testes, are lined by cuboidal or low columnar epithelium, and are located peripherally within or adjacent to the mediastinum testis. Their average size is 5 to 7 mm, and they are not palpable. Ultrasound examination reveals peripheral, well-defined anechoic lesions with normal surrounding testicular echogenicity (Fig. 20.12). Cysts of the rete testis are often accompanied by cysts or other abnormalities of the epididymis. The tubules of the rete testis may become ectatic or develop multiple small cysts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree