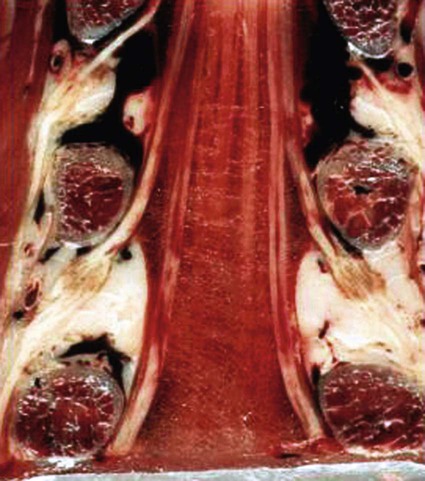
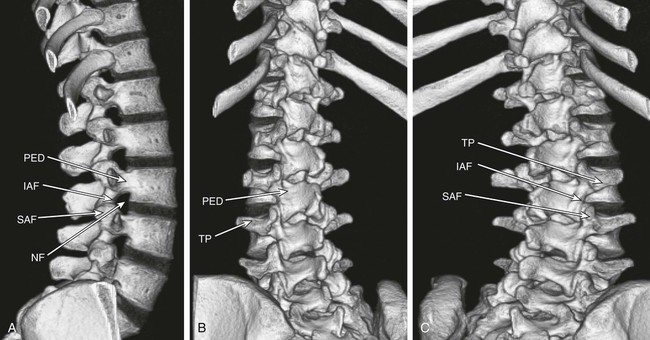
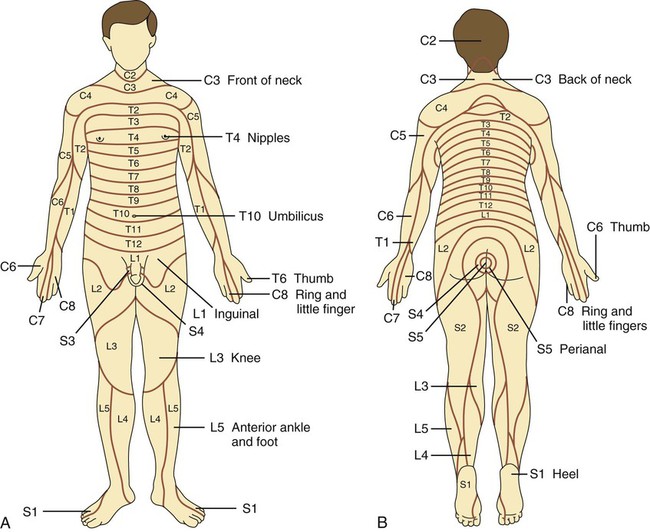
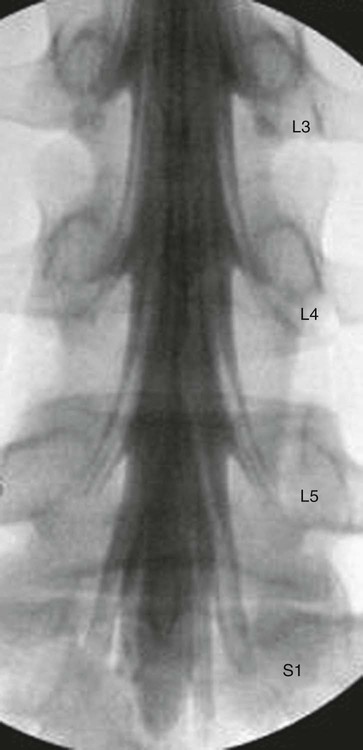
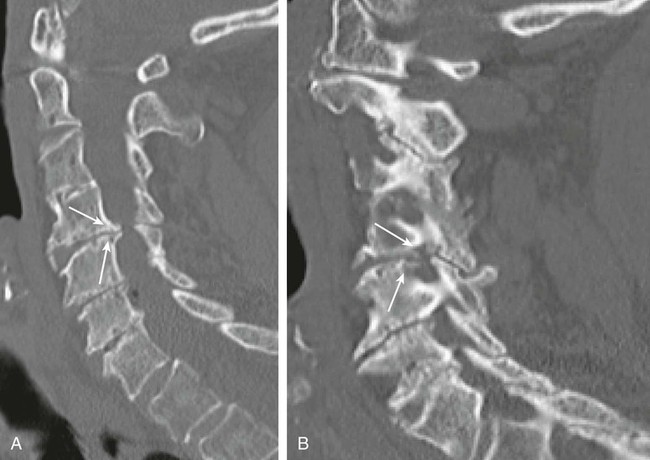
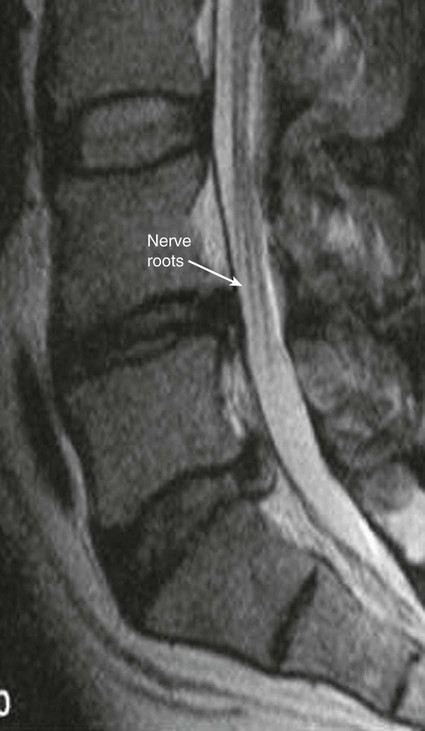
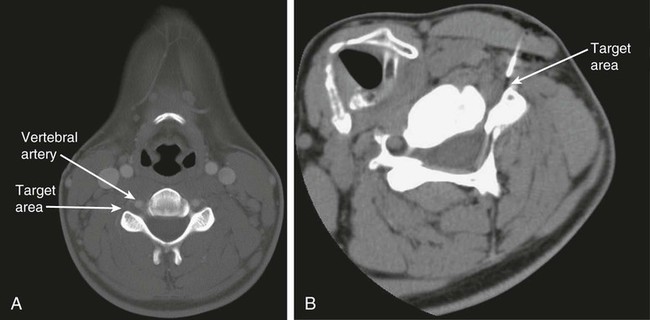
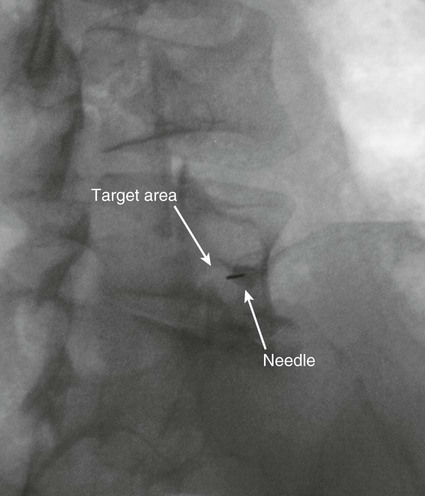
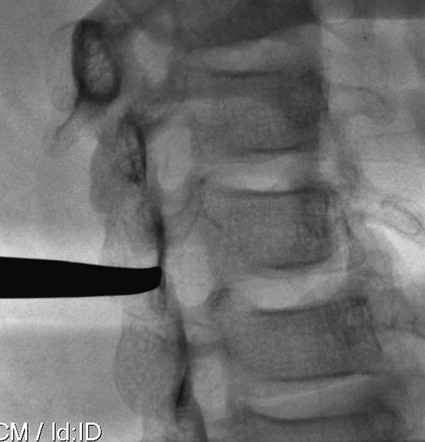
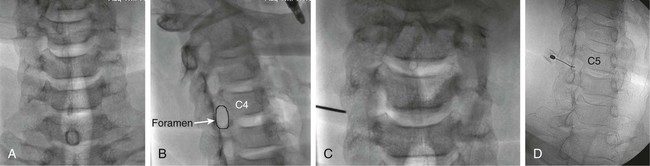
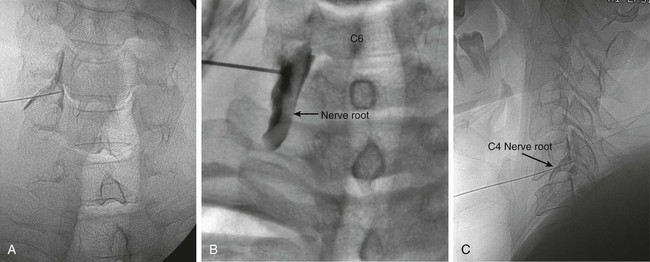
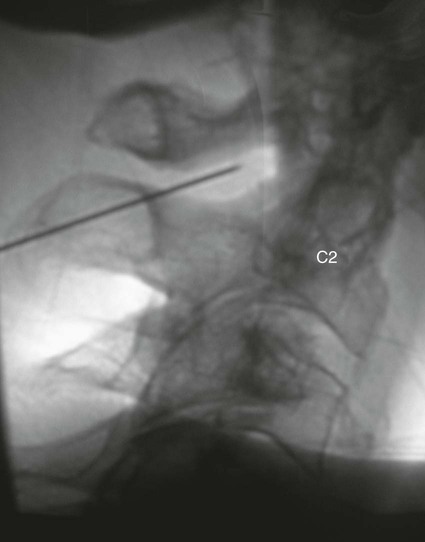
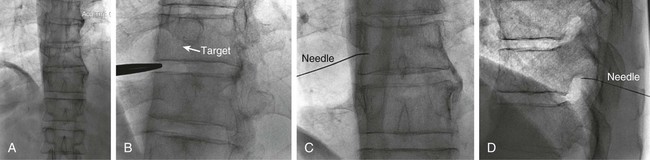
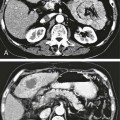
The practice of recognizing and understanding dermatomal patterns has given practitioners the opportunity to better characterize pain and analyze the process of radicular pain. The understanding and application of techniques that interrupt pain from the neck, thorax, visceral organs, or lower extremities that can be adequately blocked at the spinal level continues to evolve.1 As we better characterize pain patterns and as this process is readily adopted into clinical practice, the awareness of its efficacy will be more pervasive.2 The brain continues as the spinal cord to T12 or L1 and is protected by the meninges and the bony vertebral column. The spinal meninges covering the cord is composed of the pia mater, arachnoid, and dura mater. Protection of the spinal cord is formed from 33 specialized vertebrae. The size of the central canal, the size of the alae, and curvature of the bones give the vertebrae functional ability and provide protection function to the cord and nerve roots (Fig. 163-1). The neural foramen is the passageway for the spinal nerve as it exits the central canal. The anterior border of the foramen is framed by the uncovertebral joint and vertebral body. The pedicle of the vertebral body above supplies the roof, and the pedicle and lamina of the vertebrae below supplies the floor. The posterior aspect of the neural foramen is framed from the inferior and superior articular facets of the adjoining vertebrae (Fig. 163-2). The dorsal and ventral rootlets of the cord are covered by pia and arachnoid. Beyond the neural foramen, the pia and arachnoid form the perineural epithelium covering of the peripheral nerve. The dura extends primarily along the dorsal surface of the rootlets and extends lateral to the point where the anterior and posterior rootlets fuse to form the ganglia. This termination of dural tissue forms a small sleeve. The sleeve is of variable lengths.3 The sleeve is pierced by arteries, veins, and lymphatics that communicate with the underlying subarachnoid space. The anatomy of the nerve fiber, its diameter, and myelination can determine its physiologic response to anesthetics.4 The myelination and diameter of the nerve are important in this process. Nerve permeability regulates the readily available amount of anesthetic for blocking neural transmission. The amount of anesthetic, such as lidocaine, around the nerve root also affects its neural blockade activity. In addition, pH can enhance permeability of the perineural membrane. Local anesthetics are weakly acidic. These molecules are more stable at the acidic level, but the neutral anesthetic molecule passes across the membrane more readily.5,6 Therefore, raising the pH with small aliquots of sterile sodium bicarbonate prevents significant dilution and is beneficial in creating the alkalized form of the anesthetic.7 Pain from the zygapophyseal joint is referred to the adjacent paraspinal tissues. The pattern and potential distribution of perceptive pain in the cervical spine have been defined by Bogduk and Marsland, and in the thoracic spine by Fukui et al.8,9 The lumbar spine and paraspinal and adjacent structures produce perceptive pain from the low back, buttock, and thigh areas. However, the lower lumbar medial branch nerves may convey pain that is perceived as low as the knee and occasionally the calf. The location and distribution of pain can be diagrammed on a dermatomal map (Fig. 163-3). This valuable information can assist in determining whether single or multilevel disease is present. Recording factors such as time of day and response to aggravating or relieving factors helps characterize pain. An organic pain map has considerable diagnostic potential. It has a high degree of validity in the assessment of neurogenic pain and dysfunction and is reliable in showing concordance with the physical examination.10 How pain is influenced by functional ability, mechanical loading, bending, sitting, standing, driving, or rest can further the evaluation. Nerve roots cover a defined dermatome in most individuals, but the specific distribution or patterns are individualized. There is, however, considerable overlap in nerve root distribution. In the cervical region, the map is better defined (Fig. 163-4). In the thoracic region, the pattern of the thoracic nerve roots is segmental and covers a band or horizontal distribution. In the lumbar and sacral region, there is a more extensive distribution with overlap along the back and involvement of the buttocks and leg to the foot. Needle electromyography (EMG) can detect waves and fibrillation potentials along muscle fibers. The motor nerve fibers innervating the muscles extend across two nerve root levels. EMG does not test sensory input. Based on the muscles involved, EMG helps predict the level of the radiculopathy. EMG detects signs of irritability as the process of denervation and reinnervation progresses from acute to chronic injury.11,12 By sampling multiple muscles, it seeks to determine the specific root or roots involved. It is estimated that the specificity of the EMG is near 85%.13 Depending on the clinical condition, diagnostic imaging may provide the best clues to the diagnosis and level to be injected. Plain film evaluation of the region of concern provides a good diagnostic tool. Plain film radiography is readily available and cost-effective when diagnosing acute back pain.14 Plain films of the cervical, thoracic, and lumbar spine may reveal loss of disc height, end-plate sclerosis, neural foramen narrowing, or bone hypertrophy. Plain films assist in ruling out other benign and malignant conditions. Plain film images of the cervical, thoracic, and lumbar spine can be obtained without concern for an implant device, sedation, or claustrophobia. Spinal instruments such as screws and plates may limit evaluation of plain films but do not produce the multiple artifacts that would be seen with CT and MRI.15 Plain film images are obtained in at least two planes, frontal and lateral. Special projections such as oblique views in the lumbar and thoracic region and pillars or odontoid views in the cervical spine are helpful in evaluating pathology and postoperative changes, including fusions.16 In addition, congenital anomalies, fractures, osteoarthritic impingement, or bone erosion may be differentiated from tumor involvement with plain film radiography. Myelograms are performed with the assistance of CT. This procedure allows access to the cerebrospinal fluid for diagnostic purposes. Intrathecal administration of iodinated contrast material allows detection of abnormalities encroaching on the spinal cord or nerve roots, including herniated disc, meningeal carcinomatosis, or spinal vascular malformation (Fig. 163-5). In patients with scoliosis, CT is the preferred imaging tool over MRI, which may have limitations with visualization of the sagittal plane (Fig. 163-6). MRI shows loss of disc height, annular tears, and intradisc hypointensity on T1- and T2-weighted images. In the presence of annular tears, sagittal T2-weighted images with fat saturation show focal high signal, and subchondral marrow shows T2 hyperintensity in the vertebral bodies abutting the disc. Central canal, lateral recesses, and neural foraminal narrowing can be seen on MRI (Fig. 163-7). Associated changes such as facet hypertrophy, degenerative spondylolisthesis, and hypertrophy of the ligamentum flavum can also be seen by MRI. These findings are correlated with the selective block to determine which level or levels should be tested or should undergo neural blockade. In patients with an uncorrectable coagulopathy, spinal injections are not recommended (Table 163-1). Spinal and paraspinal infection creates a risk of spread along the spinal canal. TABLE 163-1 Coagulopathy-Related Contraindications to Spinal Injection A spinal injection or neural blockade of the nerves to the muscles used in respiration can create a thoracic block in a patient with a contralateral pneumothorax or severe chronic obstructive pulmonary disease (COPD). In the setting of a suspected tension pneumothorax, chest trauma or COPD pulmonary clearance is recommended to avoid an intraoperative crisis.17 Because local anesthetics are infiltrated as a fluid medium around the nerves, the fluid must diffuse through layers of fibrous tissue before it reaches the axons.5 The rate of diffusion depends on adjacent scar tissue, and diffusion can be especially difficult in the setting of previous surgery. The rate of absorption by fat, uptake into the microvasculature, and local metabolism all determine the clinical potency of the injection. The final concentration that reaches the nerve is affected by these factors and by the length of nerve exposed to the drug solution. Injections in close proximity to the nerve reduces the need for diffusion and the volume of anesthetic required to achieve the desired block. Intimate proximity to the nerve root can be confirmed radiographically by CT scan or fluoroscopically. Blockade of the peripheral nerve can also be affected by drug absorption in surrounding tissues. This can be affected by use of a vasoconstrictor such as epinephrine. The rate of absorption in the vasculature is reduced, thereby leaving more anesthetic available for neural blockade and prolonging the anesthetic affect.18 Depot formulations of epidurally placed medications appear to prolong the local anesthetic effect. The slow release should also be accompanied by a decrease in the rate of systemic drug absorption and reduction of the potential for systemic toxicity.19,20 The analgesic effect of anesthetics is enhanced with corticosteroids. Steroids relieve pain by reducing nociceptive C-fiber transmission and by decreasing inflammation. Steroids inhibit the action of phospholipase A2, which releases arachidonic acid from cell membranes at the site of inflammation. Membrane injury causes accumulation of edema and release of unsaturated fatty acids. Altered membrane permeability in the setting of inflammation leads to intraneural edema and causes abnormal neural transmission.21,22 Abnormal transmission along the nerve fiber results in creation of pain. Stabilization of the neural membrane by corticosteroids can inhibit sensitization of the nerve fiber and prevent the neural discharge that results in pain creation. Corticosteroid medications have a therapeutic benefit. The recommended dose is not more than 50 mg triamcinolone or 80 mg methylprednisolone.23 Corticosteroids have been used to prolong the therapeutic effect of the diagnostic test injection. The antiinflammatory effect decreases pressure along the nerve root as swelling subsides and autologous histamine substances such as substance P are diluted away. Symptom improvement can increase the time for the patient to confirm the injection is beneficial. An injection of 0.5 to 1.5 mL of contrast material is used to outline the nerve root. The volume of injected anesthetic should mirror the injected volume of contrast material. The goal of the contrast is to not only localize the nerve root but also demonstrate the extent of the spread of medication and perhaps avoid injection into the epidural space. Most injections are performed using fluoroscopic guidance, but CT can also be used for needle guidance.24 Its benefit is best seen in the cervical region where the avoidance of major blood vessels is critical (Fig. 163-8). The approach taken with the use of CT will be a direct axial one unless the CT scan gantry is angled. This approach allows the practitioner to avoid the primary vasculature of the vertebral and carotid arteries as well as the large branches of the subclavian artery, especially the superior thyroidal artery near the base of the neck. There are multiple small muscular branches of the vertebral artery that may not be avoidable, but recognition of their presence if bleeding develops as the vertebral artery is approached can be helpful. However, vigilance for the spinal artery during a cervical injection is important to avoid major complications. The precise location of a tortuous vertebral artery can be localized, especially near the skull base and at the base of the neck, before entering the foramen transversarium (Fig. 163-9). In the setting of a neck or shoulder mass, CT scan presents an excellent option to avoid direct transgression of the mass to gain access to the cervical nerve root. The basic technique is to stabilize the needle with the thumb and index finger or use a hemostat for measured advancement of the needle tip. The fluoroscope is positioned to optimize the position and alignment of the neural foramen. A gun-site or down-the-barrel projection is used when the target is the foramen. The goal is perineural placement of the needle (Fig. 163-10). To avoid puncture of the nerve root, the advancing needle tip is also visualized in the lateral and frontal projections. Ideal needle placement depends on the level of the spine being treated. In the cervical spine, the needle tip is placed posterior for the foramen (Fig. 163-11). As the needle tip nears the neural foramen, care should be taken to avoid piercing the nerve root and vertebral artery. Success of the injection is not influenced by the length of the spinal needle bevel. In comparison with long-bevel needles, short-bevel needles do not reduce the rate of vascular injection.25 For injection of the cervical spine, the needle end target is placed near the upper outer aspect of the foramen at the 10 o’clock position on the right and at the 2 o’clock position on the left of the patient. The patient can be placed in an oblique position, or the image intensifier is maneuvered to open the neural foramen and create the widest circle (Fig. 163-12). The nerve exits the foramen anterolaterally and inferiorly en route to form segments of the brachial plexus. The needle should be directed to engage the lip of the foramen posteriorly. The vertebral artery commonly lies anterior to the nerves and passes through a channel or foramen transversarium. Posterior to the needle are the articular facets (Fig. 163-13). For the injection of the C2 nerve, a direct lateral or posterior approach is taken. The pathway is centered in a rectangle created between the spinal laminar line posteriorly and the odontoid anteriorly. The superior border is framed by the lamina of C1, and the floor is created by the lamina of C2. Using a posteroanterior projection, the needle tip is advanced until it passes the lateral third of the facet joint (Fig. 163-14). Oftentimes the patient’s mouth has to be opened for adequate positioning and determination of landmarks. For injection of the thoracic spine, the needle is angled along an inferolateral approach. The image intensifier is angled so it parallels the end plates of the vertebral bodies at the level that is being approached. the foramen is approached medially from the inferior aspect of the transverse process. A curved needle is used to touch the transverse process and then walked off its edge. Just deep to the anterior aspect of transverse process, the needle will give way beyond the bone contact with the bone. The needle is advanced in a lateral to medial direction (Fig. 163-15).
Selective Nerve Root Block
Introduction
Anatomy
Tissues and Fascia
Nerves
Sources of Pain
Indications
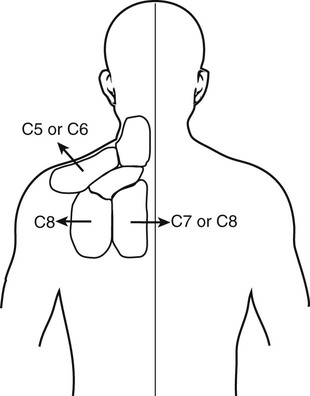
Mapping
Diagnostic Imaging
Contraindications
Baseline laboratory data contraindicating injections include:

Drugs Used for Selective Nerve Root Block
Anesthetics
Corticosteroids
Technique
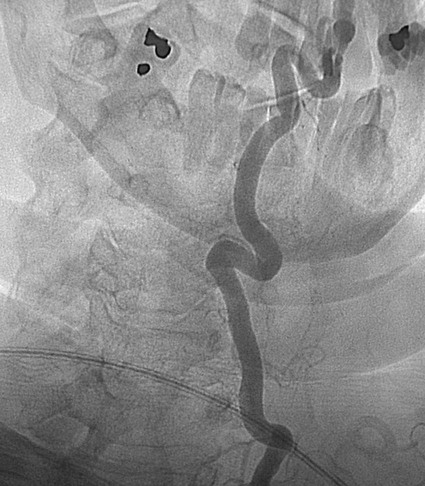
Cervical
Thoracic
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Selective Nerve Root Block