The typical challenges a radiologist confront when evaluating a patient with a shunt are to decide the location, type, and size of the defect, as well as rim size and associated anomalies, and to detect signs of right ventricular flow and pressure overload. These questions can be answered for the most part with echocardiography. The use of magnetic resonance imaging (MRI) and computed tomography (CT) is growing in these patients because of the excellent anatomic depiction with these techniques and the reliable quantification of the shunt with MRI.
Physiologic and morphologic separation between the systemic (arterial) and pulmonary (venous) circulation is essential for the normal function of the cardiovascular system. Abnormal communication between these two systems results in shunting of blood from the high-pressure system (arterial) to the low-pressure system (venous), thereby creating a left-to-right shunt. The direction and magnitude of shunting are determined by the size of the defect and the relative compliance of the ventricles. Small defects are associated with a small shunt volume and no hemodynamic sequelae. Conversely, large defects may be associated with a large shunt volume that causes right ventricular volume overload and heart failure. Communications between the venous and arterial systems can happen at the level of the heart, in the setting of atrial septal defects (ASDs) and ventricular septal defects (VSDs) ( Fig. 41-1 ), or at the level of the great vessels, as in the setting of a patent ductus arteriosus (PDA). Certain vascular malformations (abnormal pulmonary venous return, pulmonary arteriovenous malformations, and vein of Galen aneurysm) or vascular tumors (infantile hemangioendothelioma) can also result in similar physiologic features, although these lesions are beyond the scope of this chapter.

Shunts can be congenital (PDA, ASDs, patent foramen ovale [PFO], VSDs) or acquired (traumatic, infectious, or infarct-related VSDs). Their long-term effects relate to their size and amount of blood shunted per cardiac cycle. In cases of significant shunting (ratio of pulmonary flow [Qp] to systemic flow [Qs]) >1.5), overload of the right-side or venous system can result in increased right-sided pressures and ultimately in right-sided heart failure.
Intracardiac shunts can result from deficient morphogenesis of the cardiac septa (congenital ASDs and VSDs) or injury to the cardiac septa (myocardial infarction, trauma, or iatrogenic causes), or they can be secondary to persistence of fetal communications.
Shunting between the pulmonary and systemic circulation is necessary during fetal life to provide oxygen-rich blood from the venous system (which brings oxygen-rich maternal blood to the fetus through the umbilical veins) into the arterial system, thus bypassing the high-pressure prenatal pulmonary circulation. Persistence of fetal circulation (foramen ovale and ductus arteriosus) beyond the first months of life can result in pathologic left-to-right shunting.
Small shunts may go undetected for many years and may manifest as incidental findings on physical examination or imaging studies. Patients with larger defects can present with fatigue or dyspnea on exertion. On chest radiography, the finding of enlargement of the right-side chamber and pulmonary arteries with prominent peripheral pulmonary branches and distended veins is consistent with shunt vascularity suggestive of intracardiac shunts. Transthoracic echocardiography (TTE) is the initial diagnostic examination in the evaluation of intracardiac shunts given its widespread availability and high sensitivity. Transesophageal echocardiography (TEE) is reserved for guidance during placement of closure devices, given its invasiveness. MRI is a great tool for evaluating morphology and potentially associated anomalies and for quantification of shunts. CT imaging is an excellent tool in the morphologic analysis of cardiac shunts and their associated anomalies in patients in whom echocardiography is limited or MRI is contraindicated.
Definition of the Interatrial Septum
The definition of the interatrial septum is controversial and varies in the literature from the classic definition of a partition separating the upper chambers of the heart (atria) to the more accurate revised definition of a structure that divides the right and left atria that can be removed without exiting from the cardiac chambers. The cardiac imager, and particularly the interventional cardiologist and cardiac surgeon, must understand this concept because percutaneous or surgical treatments of pathologic conditions performed outside the “true” interatrial septum result in extension outside the cardiac chambers. From this perspective, the true atrial septum is exclusively made up of the flap valve of the fossa ovale (septum primum) and part of its bulbous anteroinferior base. The superior, posterior, and anterior rim of the fossa ovale, commonly referred to as the septum secundum, is the infolded wall between the superior vena cava (SVC) and the right pulmonary veins and is not considered part of the true interatrial septum as is commonly described ( Fig. 41-2 ).

Development of the Interatrial Septum
During early fetal life, a common atrium divides into right and left atria by the development of two overlapping structures, the septum primum and septum secundum, as depicted in Figure 41-3 .

Patent Foramen Ovale
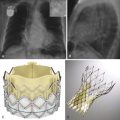
The foramen ovale is a normal communication between the right and left atrium during fetal development. A PFO results from failure of fusion between the septum primum (which forms a flaplike valve over the fossa ovale) and the septum secundum. PFOs are common, occurring in up to one third of the population. This condition is not considered a true ASD, but rather a potential communication between the two atria, given the lack of structural deficiency of the atrial septum. Atrial shunting through a PFO has been associated with cryptogenic stroke, arterial hypoxemia, migraine headaches, and decompression illness. A PFO, which is probe patent, can exist with or without detectable shunting. Shunting through a PFO can be bidirectional ( Fig. 41-4 ). Under normal physiologic conditions, the slightly higher pressures in the left atrium translate into a left-to-right shunt. During coughing, Valsalva maneuver, or pathologic conditions with increased right-sided pressures, a right-to-left shunt can result.

Treatment of PFO is controversial. Some groups prefer a conservative approach with anticoagulation, whereas others use closure devices.
Atrial Septal Defects
The different types of ASDs can be characterized by imaging techniques such as echocardiography, conventional angiography, cardiac CT, and MRI. Information regarding the morphology of these defects, their associations, and physiologic significance is needed to determine the most appropriate treatment. To understand the morphology of the different types of ASDs, the examiner must review the embryology. As with many other developmental defects, ASDs comprise a spectrum of disease with widely ranging morphologic and physiologic presentations. Before surgical correction of ASDs, morphologic assessment is needed to determine the presence of single versus multiple defects, shape, size, rim size, and associated anomalies.
Types of Defects and Imaging Appearances
ASDs are morphologic abnormalities that result in shunting at the level of the cardiac atria. The four types of ASDs can be classified as defects resulting from abnormal development of the atrial septum (ostium primum and ostium secundum [OS]) and as defects resulting in interatrial communication with normally developed atrial septum (sinus venosus and coronary sinus subtypes).
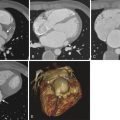
Ostium primum ASDs correspond to 2% to 3% of all ASDs. These defects are considered the mildest form of atrioventricular septal defects (AVSDs) or endocardial cushion defects (ECDs). During normal embryology, the endocardial cushions contribute to the development of the medial portions of the mitral and tricuspid valves, the portion of the atrial septum adjacent to the atrioventricular valves and the inlet portion of the ventricular septum. When the defect results from failure of fusion between the free edge of the septum primum and the atrioventricular cushions, it is termed ostium primum ASD ( Fig. 41-5 ). If the defect associates with abnormal development of the atrioventricular valves or ventricular septum, it is known as an AVSD or ECD ( Fig. 41-6 ). Because ostium primum ASDs occur secondary to the lack of fusion between the septum primum and the endocardial cushions, they are seen as interatrial communications located immediately posterior to the mitral valve annulus.


On electrocardiogram (ECG)–gated multidetector CT (MDCT) images after contrast with saline chaser, ostium primum ASDs can be seen as an abnormal communication between the right and left atria located immediately posterior to the mitral valve annulus. Depending on the size of the defect, relative equalization of contrast density in the right and left chambers can be seen. Associated enlargement of the right-sided chambers is usually present.
OS-type ASDs are the most common, accounting for 80% to 90% of ASDs. These defects are centered in the fossa ovalis of the interatrial septum. They result from excessive apoptosis of the cephalic portion of the septum primum (a large OS) or incomplete development of the septum secundum ( Fig. 41-7 ).


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree