Chapter 22 Sino-Nasal, Oral, Larynx and Pharynx Cancers
Nasopharynx
Anatomy
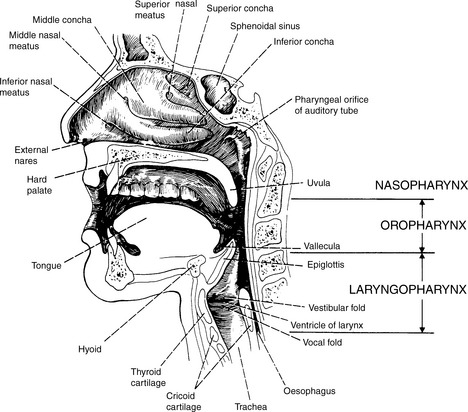
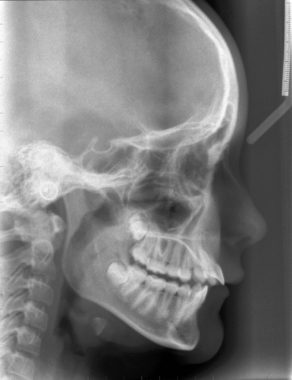
The nasopharynx is cuboidal in shape and comprises the most superior of the three pharyngeal structures. As such it has a direct communication with the nasal cavity anteriorly and oropharynx inferiorly (Figure 22.1).
Lateral walls
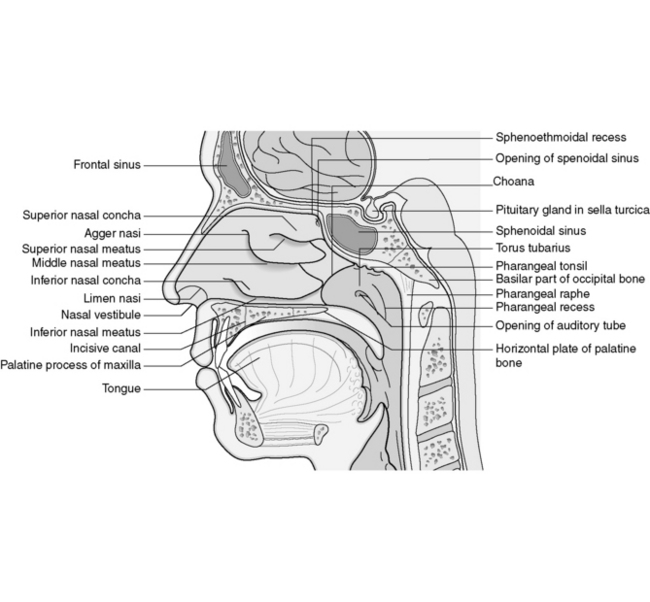
The pharygobasilar fascia forms this and the posterior wall. Within this is the opening of the eustachian tube and, more posteriorly, a deep recess called the fossa of Rosenmüller (lateral nasopharyngeal recess) (Figure 22.2).
Staging system for nasopharyngeal tumours
| T1 | Tumour confined to the nasopharynx |
| T2A | Tumour extends to the oropharynx and/or nasal cavity without parapharyngeal extension |
| T2B | Tumour with parapharyngeal extension, i.e. infiltrates beyond the pharyngobasilar fascia |
| T3 | Tumour that invades bony structures and/or paranasal sinuses |
| T4 | Tumour with intracranial extension and/or involvement of cranial nerves, infratemporal fossa, hypopharynx, orbit or masticator space |
| N1 | Unilateral (including midline) lymph node(s) ≤6 cm above the supraclavicular fossa |
| N2 | Bilateral lymph node(s) ≤ 6 cm above the supraclavicular fossa |
| N3A | Any lymph node >6 cm |
| N3B | Any node that involves the supraclavicular fossa |
Aetiology, pathology and lymphatic spread
| Type 1 | Well-differentiated keratinizing type |
| Type 2 | Moderately-differentiated non-keratinizing type |
| Type 3 | Undifferentiated type typically with an extensive lymphocytic infiltrate. |
Signs and symptoms
Patients may report headaches though other symptoms or signs will usually be readily apparent.
Nose and nasal cavity
Anatomy
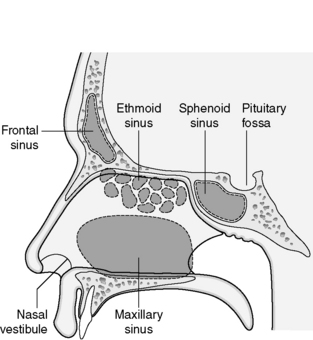
The external nose is like the tip of an iceberg with a complex array of passageways and air cavities within it that form the nasal cavity and paranasal sinuses. The hair-bearing entrance that forms the vestibule and the mucociliary escalator provides an important initial defence against the inhalation of germs (Figure 22.9).
Anterior wall
The nasal bones and cartilage that form the external nose give rise to the anterior wall.
Staging system
| T1 | Tumour restricted to one subsite of the nasal cavity |
| T2 | Tumour involves two subsites in a single site or extends to involve an adjacent site within the nasoethmoidal complex |
| T3 | As ethmoid sinus |
| T4A | As ethmoid sinus |
| T4B | As ethmoid sinus |
Treatment (see Figures evolve 22.10–22.14  )
)
Radiotherapy technique
Tumours of the vestibule and low anterior nasal fossa tumours may be treated by a direct anterior appositional electron beam, implantation with iridium wires, direct lateral photons or an anterior oblique wedged pair field arrangement (Figure evolve 22.15 ![]() ). The choice will be dictated by the extent of the clinical target volume (CTV) and the physical constraints of the particular modality as well as local expertise. The relatively superficial nature of these tumours and the shape that presents dosimetry problems usually dictates the need for some bolus material on the skin surface when using external beam treatment. The facial lymphatics may be included as a separate target volume and treated prophylactically using separate electron fields.
). The choice will be dictated by the extent of the clinical target volume (CTV) and the physical constraints of the particular modality as well as local expertise. The relatively superficial nature of these tumours and the shape that presents dosimetry problems usually dictates the need for some bolus material on the skin surface when using external beam treatment. The facial lymphatics may be included as a separate target volume and treated prophylactically using separate electron fields.
Paranasal sinus tumours
Staging system for paranasal sinus tumours
| Maxillary sinus | Ethmoidal sinus | |
| T1 | Mucosa only | One subsite |
| T2 | Bone erosion/destruction (not posteriorly) | Two subsites |
| T3 | Bone erosion/destruction (if posteriorly), involvement of the subcutaneous tissues floor and medial wall of the orbit, pterygoid fossa and ethmoid sinus | Involvement of the medial wall and floor of the orbit, maxillary sinus, cribriform plate |
| T4A | Involvement of the anterior orbital contents, skin of the cheek, pterygoid plates, infra-temporal fossa, cribriform plate, sphenoidal or frontal sinuses | As maxillary sinus |
| T4B | Involvement of the orbital apex, dura, brain, middle cranial fossa, cranial nerves (excluding the second division of the Vth cranial nerve), nasopharynx or clivus | As maxillary sinus |
Though it does not form part of the TNM staging system, division of maxillary sinus lesions into those arising from the infrastructure, that is anteroinferiorly, from lesions arising from the suprastructure which lie superoposteriorly is potentially useful. This division arises from a theoretical line drawn from the medial canthus to the angle of the mandible in a lateral plane (Ohngren’s line) (Figure 22.17).