Chapter 24 Gastrointestinal cancer
Cancer of the oesophagus
Anatomy (Figure 24.1)
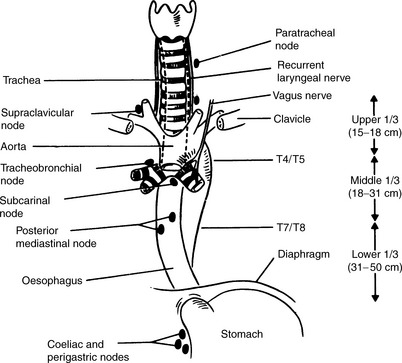
Figure 24.1 Relations of the oesophagus and lymph node drainage.
(Reproduced with permission of Arnold from Jane Dobbs, Ann Barrett, Daniel Ash, Practical Radiotherapy Planning, 3rd edn, Arnold, 1999)
Diagnostic evaluation (Tables 24.1–24.3)
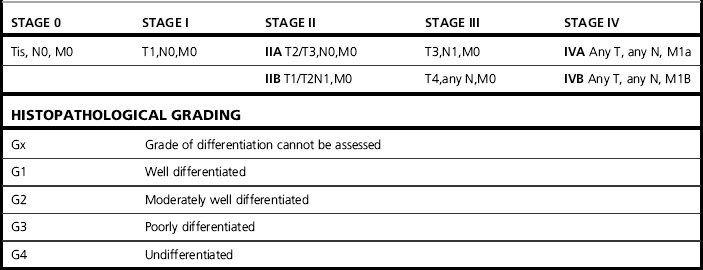
Table 24.1 Staging esophageal carcinoma
| TNM staging system applies only for esophageal carcinomas | |
| T: Primary tumour | |
| Tx | Primary tumour cannot be assessed |
| T0 | No evidence of primary tumour |
| Tis | Carcinoma in situ |
| T1 | Tumour invades the lamina propria or submucosa |
| T2 | Tumour invades the muscularis propria |
| T3 | Tumour invades the adventitia |
| T4 | Tumour invades the adjacent structures |
| N: Regional nodes (see below) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastases |
| N1 | Regional lymph node metastases |
| M: Distant metastases | |
| MX | Distant metastases cannot be assessed |
| M0 | No distant metastases |
| M1 | Distant metastases |
| For tumours of the upper thoracic oesophagus | |
| M1a Metastases in cervical lymph nodes M1b Other distant metastases | |
| For tumours of the mid-thoracic oesophagus | |
| M1a Not applicable M1b Non-regional lymph nodes or other distant metastases | |
| For tumours of the lower thoracic oesophagus | |
| M1a Metastases in coeliac axis lymph nodes M1b Other distant metastases The regional lymph nodes depend on the anatomical subsite of the oesophagus (cervical or intrathoracic) | |
| Cervical oesophagus | |
| Scalene, internal jugular, upper and lower cervical, periesophageal and supraclavicular nodes | |
| Intrathoracic oesophagus | |
| Upper periesophageal (above the azygos vein), subcarinal, lower periesophageal (below the azygos vein), mediastinal, perigastric except coeliac axis nodes pTNM Pathological classification | |
Table 24.3 Diagnostic evaluation of patients with esophageal cancer
Full blood count & renal & liver chemistry CT scan of the chest & abdomen Endoscopic ultrasound (EUS ± FNA) Bronchoscopy (in selected cases) FDG PET-CT Isotope bone scan (if indicated) |
Stomach
Staging (Tables 24.4 and 24.5)
Table 24.4 Stomach cancer TNM clinical classification