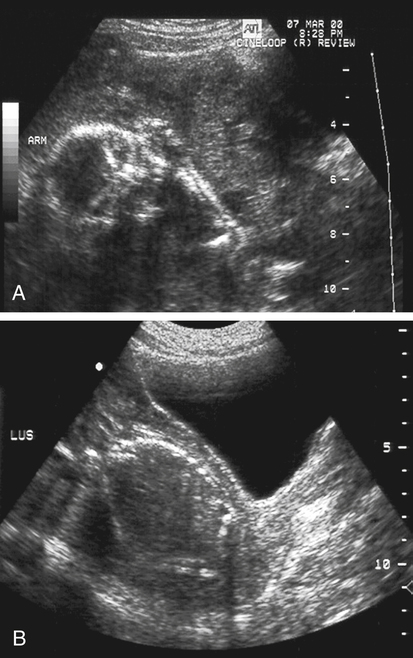
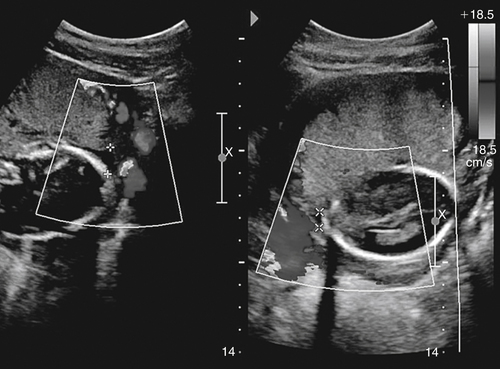
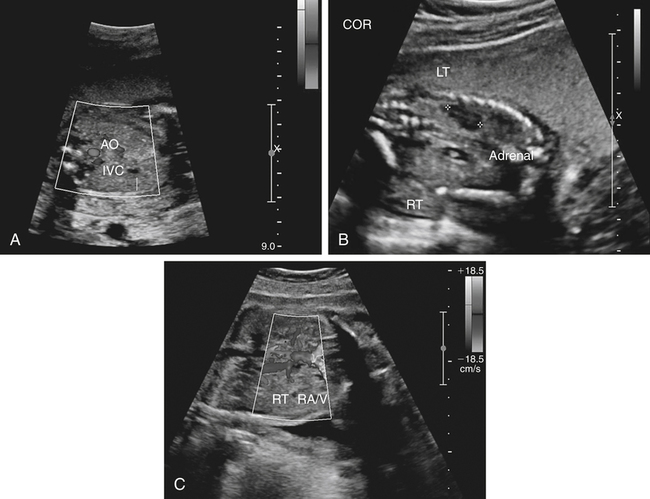
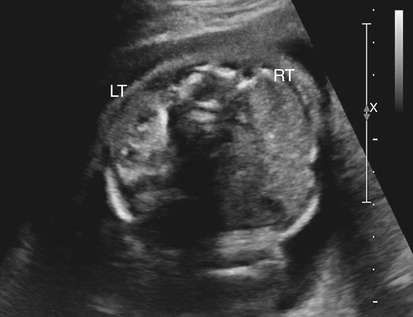
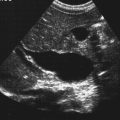
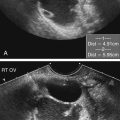
Jennie Durant and Diane Youngs • Discuss the clinical scenarios associated with a pregnancy size less than dates. • Describe the sonographic appearance and technique for a diagnosis of oligohydramnios. • Describe the sonographic appearance of common fetal renal diseases associated with size less than dates. • Describe clinical conditions, basic physiology, and sonographic findings to understand and make a diagnosis of intrauterine growth restriction. • Discuss the role of sonography in the diagnosis of premature rupture of membranes. Measurement of the uterine fundal height during every prenatal visit after 20 weeks of gestation is an integral part of obstetric care. It is a simple, safe, inexpensive, and reasonably accurate method to screen for fetal growth and amniotic fluid volume abnormalities, although it is less accurate in identifying small-for-gestational-age (SGA) fetuses. Longitudinal studies have shown that symphysis-to-fundus measurements correctly identify less than 35% of SGA fetuses.1 The standard of care is to order or perform an obstetric sonogram when the uterine fundal height is in discordance with the estimated gestational age. Oligohydramnios is a significant decrease in the normal volume of amniotic fluid. Amniotic fluid volume (AFV) results from a balance between what enters and exits from the amniotic cavity. From 20 weeks of gestation to term, the amniotic fluid is mostly made up of fetal urination and respiratory secretions, and fluid is resorbed via fetal swallowing. Alteration of these dynamics can have a significant impact on the pregnancy. The functions of amniotic fluid are numerous, including preventing fetal injury, regulating temperature, providing mobility for practicing breathing, swallowing exercises, fighting infection, discouraging contractions, and maintaining cervical length and consistency. A decrease in AFV is directly correlated with perinatal mortality and many serious morbidities.2 Oligohydramnios or anhydramnios (absence of amniotic fluid) can result from amniotic fluid loss from premature rupture of membranes (PROM) (Fig. 20-1) or can be a result of decreased fetal urinary production or excretion. Decreased urine output is associated with fetal renal anomalies in which both kidneys are dysplastic and with severe intrauterine growth restriction (IUGR) when perfusion to the kidneys is reduced. Decreased excretion is associated with urinary outlet obstruction. Ruptured membranes may be suggested with the finding of decreased amniotic fluid on sonography and a fetus that is of an appropriate size without structural anomalies. Fetal anomalies, medication or drug use by the mother (nonsteroidal antiinflammatory drugs, angiotensin-converting enzyme inhibitors, cocaine), maternal medical disease, placental insufficiency, and chromosome alterations all can explain a finding of oligohydramnios. Chronic oligohydramnios, with or without fetal abnormalities, may cause pulmonary hypoplasia, abnormal chest wall compliance, contractures, and infection.2 MVP involves a survey of the entire amniotic cavity and measurement of the largest vertical pocket of amniotic fluid, free of umbilical cord or fetal parts. The criteria for oligohydramnios vary, but a deepest pocket of less than 2 cm is most widely accepted.3 AFI is calculated by adding the largest vertical pocket of fluid measured from each of the four quadrants of the uterus. Measurement of pockets of fluid should be void of umbilical cord or any fetal parts. AFI should be measured with the patient supine and can be obtained in the transverse or sagittal plane orientating the transducer perpendicular to the floor. To divide the uterus into four quadrants, the midsagittal line is used as the vertical landmark, and an imaginary line halfway between the symphysis pubis and the fundus of the uterus is used as the horizontal landmark. Using excessive transducer pressure can cause an inaccurate AFI assessment. It is important to use color Doppler imaging while obtaining an AFI measurement (Fig. 20-2; see Color Plate 18) because this eliminates the inaccuracy of measuring the umbilical cord instead of the amniotic fluid, especially on patients who are difficult to scan. AFI of less than 5 cm is the generally accepted criterion used to diagnose oligohydramnios, and AFI between 5.1 cm and 18 cm is considered normal.3 The ability of the described sonographic methods to detect normal AFV is high; however, abnormal AFV detection is poor, especially for oligohydramnios. Studies have compared AFI and MVP, and results indicate that AFI overestimates and MVP underestimates fluid volumes.3,4 When the diagnosis of oligohydramnios is made, detailed sonographic examination of the fetus should follow; however, evaluation of other anatomic structures may be limited because of the lack of an acoustic window. Sonographic evaluation determines if the kidneys are present and functioning, and fetal biometry and Doppler assessment can evaluate for IUGR. When major anomalies are not present, onset, duration, and severity of amniotic fluid loss are important cofactors, but the gestational age at delivery remains the overriding issue.2 Renal agenesis is the congenital absence of one or both kidneys from the complete lack of formation. The incidence rate of renal agenesis is 1 in 3000 births and 1 in 240 stillbirths.5,6 It is 2.5 times more common in male fetuses than in female fetuses. Bilateral absence of the kidneys is more common in twins than in singletons. An increased incidence is not seen with advanced maternal age or maternal medical disease. The sonographic criteria for the diagnosis of bilateral renal agenesis are (1) presence of severe oligohydramnios or anhydramnios after 14 to 16 weeks of gestation and (2) failure to visualize the fetal kidneys and the urinary bladder (Fig. 20-3, A; see Color Plate 19). After 26 to 28 weeks of gestation, preterm premature rupture of membranes (PPROM) or placental dysfunction may be the cause for the low or absent AFV. First-trimester diagnosis of renal agenesis is rare because AFV is often not reduced at that point in gestation because its volume is primarily from the placenta. The fetal kidneys may be visualized from 10 weeks of gestation with transvaginal sonography. In contrast to the fetal adrenals, which are relatively hypoechogenic, the fetal kidneys appear as bilateral echogenic masses with a similar density to that of the fetal lungs. The fetal adrenal glands have served as a source of false-negative diagnoses in cases of renal agenesis. They may appear enlarged and be confused for fetal kidneys. However, adrenal hypertrophy has been found not to occur, and the false-negative diagnosis of renal agenesis is related to a change in the normal adrenal shape and not to any enlargement of the fetal adrenal gland. In the absence of a fetal kidney, the adrenal gland, which is no longer in its transverse lie draped over the superior pole of the kidney, now is primarily in a longitudinal lie (Fig. 20-3, B). This position gives it an enlarged appearance within the renal fossa. Difficulty may arise in identifying the fetal kidneys in the presence of oligohydramnios. Color or power Doppler imaging is recommended as an adjunct in the diagnosis of bilateral renal agenesis. With correct Doppler settings, color Doppler imaging can aid in identifying the renal arteries (Fig. 20-3, C; see Color Plate 20), and renal agenesis is strongly suspected when the renal arteries are not identified. Autosomal recessive polycystic kidney disease (ARPKD) occurs in approximately 1 in 20,000 births.7 This type of polycystic kidney disease is caused by mutations in the PKHD1 gene and is characterized by nonobstructive dilations of the collecting ducts in the kidneys and hepatic fibrosis.8 Severe cases can result in perinatal death when pulmonary hypoplasia is caused by long-standing oligohydramnios. Both kidney and liver disease are progressive in most patients, requiring kidney transplantation before the age of 20 years or treatment of early-onset severe hypertension, esophageal varices, and recurrent cholangitis, or both transplantation and treatment of the aforementioned complications.8 Fetal liver changes may not be evident sonographically; however, 30% of patients with ARPKD present perinatally with enlarged kidneys.8 DNA previously collected from the first fetus affected with ARPKD is invaluable to facilitate early prenatal diagnosis in subsequent pregnancies. Typical in utero presentation of ARPKD is the finding of enlarged, echogenic kidneys with loss of corticomedullary differentiation, along with oligohydramnios (Fig. 20-4). The echogenicity of the renal parenchyma is thought to be the result of numerous, closely spaced small cysts that cannot be detected sonographically. Increased echogenicity has been identified by 12 to 16 weeks of gestation. An accurate diagnosis of ARPKD may be difficult early in pregnancy because the size of the fetal kidneys may still be normal. Evidence of renal enlargement and marked echogenicity is usually present by 24 weeks of gestation. Occasionally, the diagnosis cannot be made until the third trimester or after birth. Oligohydramnios is associated with renal dysfunction, and marked oligohydramnios is a common finding. A detailed family history, together with sonographic evaluation of the parents’ kidneys, is helpful in obtaining the correct diagnosis because both ARPKD and autosomal dominant polycystic kidney disease can manifest with oligohydramnios. Dysplastic kidneys can be of any size, ranging from small kidneys (with or without cysts) to large kidneys distended from multiple large cysts up to 9 cm in diameter. Multicystic dysplastic kidneys (MCDK) occur when parenchymal dysplasia causes renal cysts; this condition is not known to be inheritable.7 The incidence rate of unilateral dysplasia is 1 in 3000 to 5000 births. It occurs with less frequency in the bilateral form, with an incidence of 1 in 10,000.9 Prenatal diagnosis of unilateral dysplastic kidneys is variable. Bilateral MCDK is more likely to be diagnosed earlier in the pregnancy because of the associated finding of oligohydramnios. A strong association exists between MCDK and obstruction. Multicystic kidneys are usually attached to atretic ureters. A detailed examination of the fetus should be performed to identify contralateral renal anomalies (Fig. 20-5, A) or associated extrarenal anomalies, including heart, spine, extremities, face, and umbilical cord. Genetic counseling and karyotyping should be offered to rule out chromosomal abnormalities.
Size Less than Dates
Oligohydramnios
Sonographic Findings
Fetal Renal Anomalies
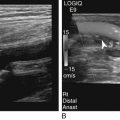
Renal Agenesis
Sonographic Findings
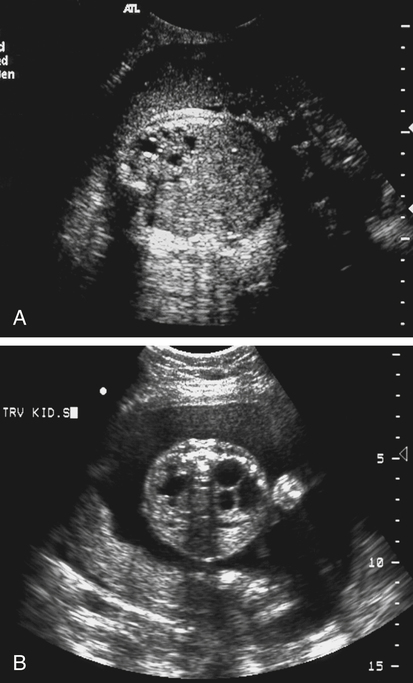
Autosomal Recessive Polycystic Kidney Disease
Sonographic Findings
Multicystic Dysplastic Kidneys
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Size Less than Dates