Abstract
This chapter describes FDG PET/CT interpretation of the skeletal system. FDG-avid osseous malignancy includes metastases, lymphoma, multiple myeloma, and sarcomas. Some osseous malignancy may not be FDG avid, such as lobular breast cancer metastases. There are many causes of osseous FDG avidity that are benign and must be distinguished from malignancy.
Keywords
FDG, PET/CT, osseous, metastases, lymphoma, myeloma, sarcoma
When approaching interpretation of osseous lesions on 18F-fluorodeoxyglucose positron emission topography/computed tomography (FDG PET/CT), it may be useful to use a 2 × 2 box divided into quadrants based on presence or absence of FDG avidity and malignant versus benign ( Fig. 3.1 ). Malignancy is often FDG avid, and thus FDG PET helps to identify malignancy that is otherwise difficult to appreciate. However, it is important to remember that not all malignancy is FDG avid, and not everything that is FDG avid is malignant. The combination of the FDG PET and CT findings often allows us to properly identify a finding as benign or malignant.

FDG-Avid Cancer
Metastases
The upper right-hand corner of the 2 × 2 box is where FDG PET provides tremendous value. FDG PET often provides superior evaluation of osseous metastases than anatomic imaging such as CT and even magnetic resonance (MR). CT requires substantial alterations in the structure of bone before the lytic or sclerotic changes allow for identification of osseous malignancy. Thus FDG PET has higher sensitivity for detection of osseous malignancy than CT in most cases.
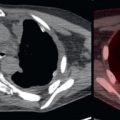
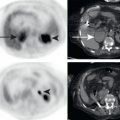
Fig. 3.2 demonstrates a patient with invasive ductal breast cancer. The patient was initially stage IIIB (locally advanced breast cancer [LABC]). LABC would be treated with neoadjuvant chemotherapy and surgery. FDG PET/CT demonstrates unsuspected distant metastases in approximately 30% of locally advanced ductal breast cancer. The identification of these previously unsuspected distant metastases increases the patient’s stage to IV (metastatic disease) and changes the treatment strategy from neoadjuvant chemotherapy and surgery to palliative systemic therapy. An example of this is seen in Fig. 3.2 . FDG PET/CT identified a previously unsuspected osseous metastasis, dramatically altering this patient’s course of treatment. There was no definite corresponding abnormality on CT, thus the FDG PET demonstrated CT occult malignancy. Some may suggest that the osseous lesion could have been detected by 99m-technetium methylene diphosphonate (MDP) bone scan; however, in this case a bone scan had been recently performed and failed to identify the osseous metastasis. This is a demonstration of how FDG PET/CT may replace the use of CT with bone scan for systemic staging of patients with breast cancer.
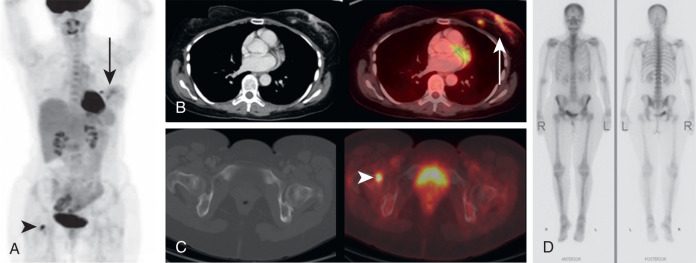
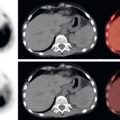
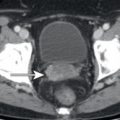
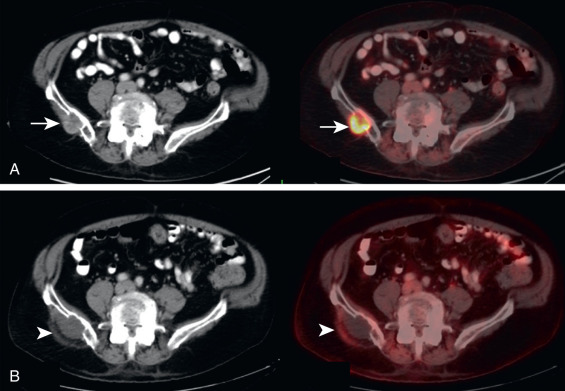
It is not uncommon for FDG PET to identify CT occult osseous metastases. Thus round foci of FDG avidity within a bone, without a CT correlate, should be considered suspicious for osseous metastases until proven otherwise ( Fig. 3.3 ). Even small FDG-avid foci within the bone without CT correlate are significant ( Fig. 3.4 ).
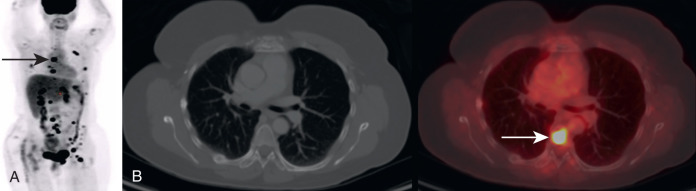
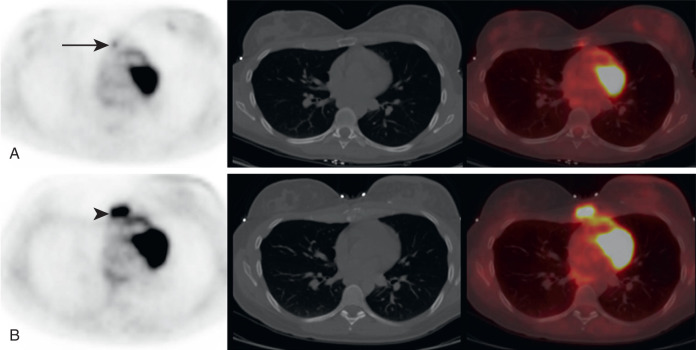
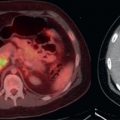
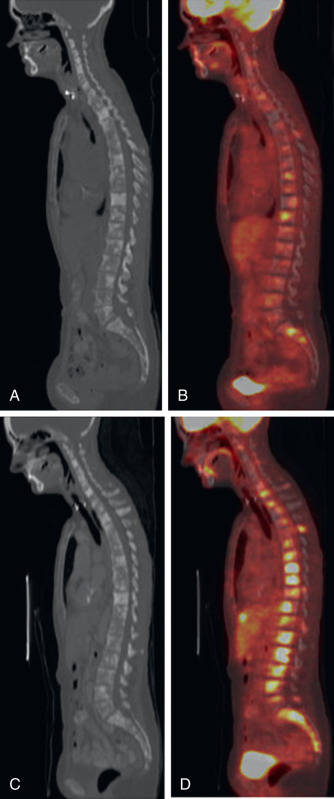
In addition to early detection of osseous metastases, FDG PET greatly adds to the follow-up of osseous metastases. It may be very difficult to determine whether osseous disease is increasing, decreasing, or unchanged on anatomic imaging, whereas FDG PET more accurately demonstrates the extent of active malignancy ( Fig. 3.5 ).
Lymphoma
Similar as for osseous metastases, FDG PET often provides greater sensitivity for detection of osseous lymphoma than does anatomic imaging. After the diagnosis of lymphoma, common staging studies include FDG PET/CT and bone marrow biopsy. Bone marrow biopsy is usually performed in the posterior pelvis and may detect osseous lymphomatous involvement in the absence of focal FDG avidity. However, bone marrow biopsy samples only one specific osseous site, and thus body imaging with FDG PET may demonstrate foci suspicious for lymphomatous involvement in the absence of positive bone marrow biopsy. In this case, FDG PET/CT may provide localization for a potential biopsy site to pathologically document osseous lymphoma. Some studies have suggested that focal osseous FDG avidity that is suspicious for osseous lymphoma may be taken as evidence of osseous lymphoma without pathologic proof. After diagnosis, FDG PET/CT provides valuable information about response to therapy in osseous lymphoma, similar to other sites of lymphomatous involvement. The Lugano Criteria state that (for initially FDG-avid lymphoma) posttreatment reduction of FDG avidity to less than liver background represents a complete response to treatment, whereas residual FDG avidity greater than liver background is suspicious for residual active lymphoma.
Multiple Myeloma
Traditional staging of multiple myeloma was performed with skeletal survey radiographs; however, the use of FDG PET/CT and whole-body MR is increasing. The 2014 International Myeloma Working Group Consensus now includes FDG PET/CT criteria for diagnosis of multiple myeloma. Interestingly, it is not the presence of FDG-avid osseous lesions, but rather the presence of osteolytic osseous lesions on the CT component that is used to make the diagnosis of myeloma. Osseous FDG avidity needs to correlate with a lytic lesion on CT, signal abnormality on MR, or biopsy-proven myeloma to be used for myeloma diagnosis. At diagnosis, multiple myeloma demonstrates a range of FDG avidity, extending from not appreciably avid to markedly FDG avid. For myeloma with appreciable FDG avidity at diagnosis, follow-up FDG PET/CT can be used to demonstrate treatment response. Changes in FDG avidity following treatment on FDG PET usually occur more rapidly than do changes apparent on CT or MR anatomic imaging.
Primary Osseous Sarcomas
FDG PET has little value in the diagnosis of primary osseous sarcomas, such as osteosarcoma or Ewing sarcoma. These sarcomas are far better diagnosed by radiograph, CT, or MR imaging. FDG PET/CT could be used for systemic staging of osseous sarcomas because sites of osseous metastases may be detected by whole-body imaging. Lung metastases, a common site of metastatic disease from primary sarcomas, are probably better evaluated by dedicated chest CT imaging than by FDG PET/CT because dedicated chest CT imaging can be performed with breath-hold, which increases the ability to detect small pulmonary lesions. The CT component of FDG PET/CT is typically performed during free breathing, for the CT component to be registered to the FDG PET component (which may take 20 to 30 minutes to acquire so breath-hold is not possible), which limits visualization of small pulmonary lesions.
Not FDG Avid and Not Cancer
The lower left-hand corner of the 2 × 2 box demonstrates an additional area of value for FDG PET. Following treatment, it is often more difficult to determine the extent of treatment response on anatomic imaging compared with FDG PET. Indeed, treatment response cannot be confused with new malignancy on CT. For example, in Fig. 3.6 , a patient with breast cancer at baseline demonstrates FDG-avid osseous lesions that are occult on CT. Following treatment, the FDG-avid foci resolve, but there are new sclerotic osseous lesions on the CT. Because osseous metastases in patients with breast cancer may be lytic or sclerotic on CT, the appearance of new sclerotic osseous lesions on the follow-up examination could be mistaken for new osseous metastases on the CT. However, the correlating FDG PET data demonstrate that the osseous metastases have been successfully treated, and the new sclerotic lesions on CT are due to treatment response rather than to new osseous malignancy.

It is important to remember that osseous lesions often become more sclerotic on CT following successful treatment, and this should not be confused with disease progression. Fig. 3.7 demonstrates a patient with initially lytic osseous lesions that become more sclerotic following treatment. Perhaps the most confusing scenario is when the osseous malignancy is initially sclerotic and the lesions become larger or more sclerotic following treatment ( Fig. 3.8 ). Whether the osseous metastases are lytic, sclerotic, or occult on CT, changes in FDG avidity on FDG PET are almost always more valuable in determining treatment response than are changes on CT.
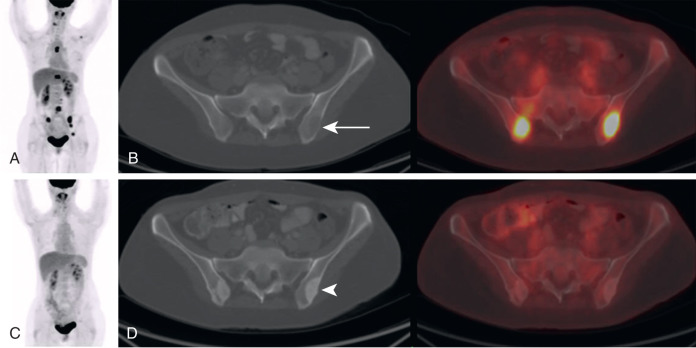
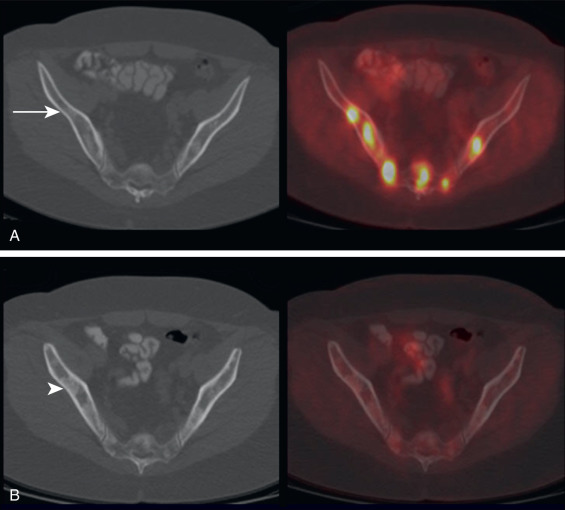
Another scenario in which FDG PET can provide superior assessment of treatment response is following interventional procedures such as ablations ( Fig. 3.9 ). Following an ablation, the treated lesion on anatomic imaging is often larger than the original malignancy. This is because a rim of tissue around the malignancy is also ablated to increase the likelihood that the margins of the tumor have been killed. The resulting CT lesion following an ablation may be confused with increased malignancy, particularly if the history of an interval ablation was not provided. On the other hand, the resolution of FDG avidity within the lesion on FDG PET more accurately depicts successful treatment.
Not FDG Avid but Cancer
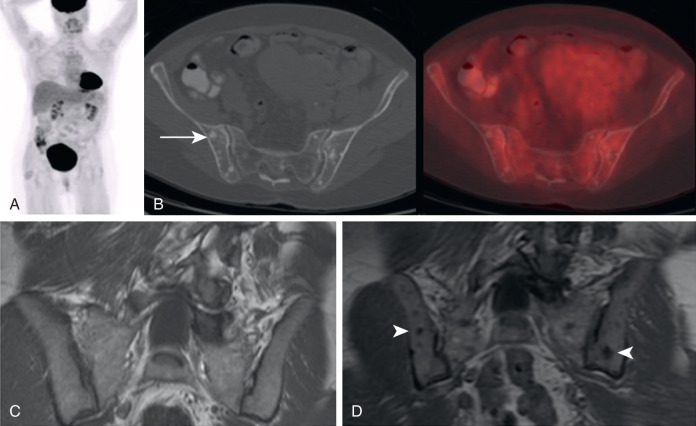
The lower left-hand corner of the 2 × 2 box is an area of concern for correct interpretation of FDG PET/CT. Because FDG PET often demonstrates malignancy that may not be apparent on CT, it is sometimes forgotten that not all malignancy is FDG avid. It is the opinion of the author that it is still the responsibility of the interpreting physician to evaluate the CT component of FDG PET/CT for evidence of malignancy that is not apparent on the FDG PET. For the skeleton, that means being aware that some histologies of malignancy are more likely to produce non–FDG-avid osseous metastases, such as lobular breast cancers. Lobular breast cancer is a histology of malignancy that is clinically and molecularly distinct from the more common ductal breast cancers. Lobular breast cancers are more difficult to visualize on all current imaging modalities, including mammogram, ultrasound, MR, and FDG PET. However, the reaction to the malignancy in bone may produce osseous lesions that are not apparent on FDG PET. Thus the appearance of new osseous lesions on CT following a diagnosis of malignancy may represent osseous metastases, even if lacking FDG avidity ( Fig. 3.10 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree