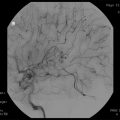
Fig. 39.1
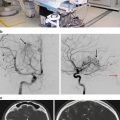
(a, b) Preoperative T1-weighted post-gadolinium MRI (a axial view, b coronal view) demonstrating a glomus jugulare tumor in the right jugular foramen (white arrow). An extended far lateral transjugular infratemporal fossa approach was performed with gross total resection of the tumor. (c, d) Postoperative T1-weighted post-gadolinium MRI (c axial view, d coronal view) shows complete resection of the tumor
Microsurgical resection remains the treatment of choice for glomus jugulare tumors [18, 19]. Early attempts at surgical resection of these tumors were fraught with poor tumor control, high rates of recurrence, and significant morbidity and mortality. As a result, over time, several approaches for microsurgical resection of glomus jugulare tumors have been described, either as single or staged operations. These approaches include mastoid-neck [20], mastoid-neck with limited facial nerve mobilization [20], infratemporal fossa type A and B [20–27], posterior fossa [23, 24], subtemporal-infratemporal [25], retrosigmoid [25], extreme lateral transcondylar [25], posterolateral [28], transmastoid-transcervical [22], combined infratentorial and posterior fossa [29], petro-occipital trans-sigmoid [30], combined transmastoid retro- and infralabyrinthine transjugular transcondylar transtubercular high cervical [31], infralabyrinthine retrofacial [32], and various combined approaches.
The primary goal of the microsurgical management of glomus jugulare tumors is curative GTR. Recent studies have repeatedly shown excellent rates of GTR of these lesions and surgical control rates have consistently remained high in numerous studies [32–34]. Surgical control rate (also referred to as tumor control rate) is defined as complete tumor elimination with no evidence of residual or recurrent disease throughout the follow-up period, including patients who have multiple surgeries. Recently, many studies have been published that have corroborated this evidence. A systematic review of seven microsurgery case series with a total of 374 patients showed 88.2 % GTR of tumors during the initial surgery and a surgical control rate of 92.1 % [17, 20, 25, 27, 29, 30, 35]. A meta-analysis by Ivan et al. [34] showed that GTR was achieved in 351 (81 %) of 433 patients during the initial surgery for glomus jugulare tumors with an 86 % surgical control rate. In a retrospective study by Borba et al. [32] of 34 patients who underwent microsurgery for glomus jugulare tumors, GTR was achieved in 91 % with a surgical control rate of 94.2 % [32]. In a more recent literature review by Suarez et al. [33], 226 patients with glomus jugulare tumors demonstrated a surgical control rate of 93.3 %.
Despite the relatively high success rate, postoperative complications, such as cranial nerve deficits, are significant causes of morbidity in patients with glomus jugulare tumors that are treated with microsurgery. Due to their location near the cerebellopontine angle, CN VII, VIII, and the lower cranial nerves are primarily affected during these operations. The most common postoperative presentations related to cranial nerve dysfunction reported in the literature include facial palsy, hearing loss, vocal cord paralysis resulting in hoarseness, dysphagia, and decreased gag reflex resulting in aspiration. In most cases, these cranial neuropathies were transient. Thus, it is clear that cranial nerve preservation during resection of glomus jugulare tumors is a key factor in reduction of postoperative morbidity in these patients. In addition, prevention of postoperative CSF leak is another important challenge that is encountered during open microsurgical treatment of glomus jugulare tumors. CSF leak has been reported in more than half the cases presented in the literature [20–24, 26, 29, 32, 34, 36].
More recently, the use of Gamma Knife, linear accelerator (LINAC), and CyberKnife SRS has been increasing for both primary and secondary treatments of these tumors largely due to the advantage of avoiding high rates of morbidity. These tumors can serve as ideal candidates for treatment with SRS because they are well demarcated on MRI and rarely invade the brain, allowing a steep dose decrease at the margin [37]. A recent meta-analysis of 19 studies describing the outcomes of patients with glomus jugulare tumors who were treated with SRS as a primary treatment exhibited an average tumor control rate of 97 % with minimal morbidity [38]. Thus, SRS should be considered as a primary treatment in patients who cannot tolerate surgery (i.e., advanced age and/or medical comorbidities), patients who have undergone contralateral jugular foramen surgery, and for smaller tumors that do not exhibit mass effect. SRS is an important adjuvant therapy for residual or recurrent tumor that is not amenable to surgical resection.
The method of treatment of glomus jugulare tumors must be individualized and tailored based on the tumor size, location, extent of intracranial extension, as well as the patient’s overall surgical risk. Early and aggressive surgical resection is ultimately favored for small tumors in younger patients in order to offer the patient the best chance for preservation of neurological function and prevention of future deterioration given that the patient is an appropriate surgical candidate [18, 21–23, 32, 36]. Tumors with significant intracranial extension exhibiting mass effect or brainstem compression and those that are malignant glomus jugulare tumors should be removed surgically.
Chordomas
Chordomas are a rare neoplasm derived from undifferentiated notochordal remnants in the axial skeleton, accounting for 1–4 % of all bone malignancies and 17.5 % of malignancies of the axial skeleton [39, 40]. Chordomas of the skull base typically arise from the clivus (Fig. 39.2) and have an incidence of approximately one per two million population, representing approximately 25–39 % of all chordomas [41–43]. While these tumors are histologically low grade, they are highly infiltrative, associated with a large tumor burden at diagnosis, and exhibit high rates of recurrence, making them very difficult tumors to manage clinically [44]. Without intervention, these tumors insidiously progress and invade surrounding structures, ultimately resulting in significant disability and death [45].


Fig. 39.2
(a, b) Preoperative sagittal T2 (a) and T1-weighted post-gadolinium (a) MRI demonstrating a clival chordoma with brainstem compression. An endoscopic endonasal transclival approach was performed resulting in near-total resection of the tumor. (c, d) Postoperative sagittal T2 (c) and T1-weighted post-gadolinium (d) MRI shows decompression of the brainstem and basilar artery
Clival chordomas typically present with cranial nerve palsy secondary to tumor compression [44], and involvement of the sella turcica by these tumors may also result in endocrinopathies [46]. Less commonly, they may present acutely with epistaxis or intracranial hemorrhage [47, 48]. The mainstay of treatment for skull base chordomas is surgical resection followed by postoperative adjuvant radiotherapy [49]. However, due to their proximity to critical neurovascular structures, the balance between obtaining maximal tumor resection and limiting significant postoperative morbidity from extensive resection has remained a topic of significant debate [50]. A recent meta-analysis of 23 observational studies conducted by Di Maio et al. [49] suggested that radical resection was the most promising method of treatment for improving the progression-free survival and overall survival in patients with skull base chordomas. They found progression-free survival rates of 85 % and 47 % with complete resection compared to 50 % and 25 % with incomplete resection at 5- and 10-year intervals, respectively. Furthermore, patients treated with complete resection had overall survival rates of 95 % at both 5 and 10 years, whereas patients treated with incomplete resection had overall survival rates of 71 % and 53 % at 5 and 10 years, respectively. Using a Kaplan–Meier analysis, they found a 3.83-fold increase in the risk of recurrence and a 5.85-fold increase in the risk of death at 5 years for patients treated with incomplete resection when compared to those treated with complete resection.
Currently, a number of surgical approaches exist for accessing clival chordomas depending on the size and location of the tumor. Staged procedures using more than one approach have also been advocated by some authors for treatment of exceedingly complex lesions [51, 52]. In a study of 71 patients, Sen et al. [42] described their rationale for approaching these tumors using midline anterior approaches, including extended subfrontal, transoral, transmaxillary, transmandibular and more recently, endoscopic endonasal approaches, for central extradural tumors [42]. For tumors extending into the cavernous sinus, surrounding the internal carotid artery, or involving the vertebrobasilar system and the brainstem, they recommend using a lateral skull base approach, suggesting that it allows for better control of the vessels and better separation of the brainstem–tumor interface [42]. Recently, the endoscopic endonasal approach has become a favorable approach among many neurosurgeons [42, 53]. The utility of this approach in treating chordomas is largely due to the fact that these tumors are typically midline and remain extradural, offering a low risk of CSF leak [53]. In an analysis of 37 studies including 766 patients, Komotor et al. [53] found that the endoscopic endonasal approach offered significant advantages over open microsurgical approaches. These include significantly higher rates of gross total resection (61.0 % vs. 48.1 %), fewer cranial nerve deficits (1.3 % vs. 24.2 %), lower rates of meningitis (0.9 % vs. 5.9 %), less overall mortality (4.7 % vs. 21.6 %), and fewer cases of tumor recurrence (16.9 % vs. 40.0 %). However, their conclusions were limited by selection bias and a shorter length of follow-up for endoscopically treated tumors. Nevertheless, they recommend that the endoscopic endonasal route is at least as efficacious as existing microsurgical techniques and suggest that it be highly considered for patients with small midline tumors (<4 cm diameter or <35 cm3 volume) with minimal lateral extension or carotid artery involvement [53].
Adjuvant postoperative radiation therapy is a widely accepted adjunct therapy for the management of residual tumor following incomplete surgical resection, and has also been suggested to have some utility in cases of gross total resection [49, 51, 54]. Proton-beam radiotherapy is currently the most widely accepted form of adjuvant treatment for skull base chordomas, with studies suggesting that it provides improved progression-free survival when compared to conventional radiotherapy [55]. However, there is a significant dearth of data comparing proton-beam radiotherapy with other modalities, such as SRS. Furthermore, multiple studies have suggested that there is no difference between the types of adjuvant radiotherapy that are offered [49, 56]. Ultimately, we recommend that all patients with skull base chordomas receive aggressive surgical resection as the primary treatment and be considered for adjuvant postoperative radiation therapy or SRS for residual disease, with the type of radiotherapy left to the discretion of the treatment team based on the available options.
Chondrosarcoma
Chondrosarcomas of the skull base account for approximately 0.15 % of all intracranial tumors and 6 % of all skull base neoplasms [57, 58]. It is postulated that these tumors arise de novo from chondrocytes within the endochondral cartilage remnants of the petrous portion of the temporal bone and areas of the petro-occipital, spheno-occipital, and spheno-petrosal synchondroses; however, other authors suggest that some of these tumors may arise as a result of the metaplasia of mature fibroblasts [58–61]. Chondrosarcomas tend to present in the fourth and fifth decades and are associated with Ollier’s disease, Maffucci syndrome, Paget’s disease, and osteochondromas [58]. They are typically divided into four groups based on histological appearance, including conventional, dedifferentiated, clear cell, and mesenchymal subtypes. Most skull base chondrosarcomas fall under the conventional or mesenchymal classifications, with conventional subtypes being far more common. Fortunately, conventional subtypes also have a better prognosis than mesenchymal subtypes, with lower rates of both recurrence (16 % vs. 63 %) and 5-year mortality (6 % vs. 54 %) [58, 62]. Additionally, these tumors are histologically stratified into a three-tiered grading system. Grade 1 tumors have the least malignant properties and lowest rates of recurrence, with better long-term survival, while Grade 2 and Grade 3 tumors possess progressively more malignant histological characteristics and are associated with worse outcomes [62, 63]. It is important to recognize these factors when determining the appropriate treatment plan, as high-grade and non-conventional lesions may require more aggressive treatment to obtain adequate tumor control. Furthermore, it is important to distinguish these tumors from chordomas, as chondrosarcomas are associated with a lower rate of recurrence and better long-term survival [64–66]. This distinction can be made by staining for markers such as epithelial membrane antigen and cytokeratin, which will stain positive in the case of chordoma, but negative for chondrosarcomas [52].
Chondrosarcomas tend to compress or invade surrounding neural structures as they progressively enlarge. Most commonly, patients will present with diplopia and headache secondary to tumor enlargement and compression of cranial nerves III, IV, and VI [52, 58, 66]. Less frequently, the tumor may also affect cranial nerves II, V, and VIII, resulting in visual loss, trigeminal pain or numbness, and hearing loss [52, 66]. Unfortunately, in many cases, complete surgical resection is not attainable due to the extensive involvement of surrounding critical structures, such as the cavernous sinus and internal carotid engulfment [66]. Therefore, decompression of neural structures, blood vessels, and the brainstem by tumor debulking is typically the primary goal, with additional resection being attempted only if the risk of injury to other surrounding structures is minimal [66]. In an analysis of tumor recurrence and outcomes of 560 patients with cranial chondrosarcomas conducted by Bloch et al. [58, 62], they found that patients treated with GTR had similar rates of recurrence-free survival compared with patients who received incomplete resections followed by adjuvant radiation therapy. Furthermore, they found that recurrence rates were 44 % vs. 9 % and 5-year mortality rates were 25 % vs. 9 % for patients treated with surgery alone and those treated with surgery followed by radiation therapy, respectively. Ultimately, they suggest that less aggressive resections may be appropriate in many situations in order to minimize the potential morbidity from surgery, especially in low-grade and conventional-type tumors.
The surgical approach to skull base chondrosarcomas varies greatly depending on the location of the tumor and the degree of involvement of surrounding structures. In patients with large complex tumors, a staged method using multiple surgical approaches to access various surgical corridors may be warranted [52]. Wanebo et al. [66] illustrated a variety of approaches determined by tumor location. For tumors of the paraclinoid region and upper half of the clivus, they recommend employing a pterional approach with the addition of an orbitozygomatic osteotomy, as this provides a more extensive basal exposure [66–68]. For tumors of the lower half of the clivus, they advocate use of a retrosigmoid approach. If there is widespread soft-tissue involvement or lateral extension of tumor, they elect for a multidisciplinary strategy including the addition of transfrontal and transmaxillary approaches. Since these tumors frequently appear in the midline, numerous authors have also advocated for the use of endoscopic endonasal approaches [69–71]. Proponents of this approach suggest that the endoscope offers the benefits of minimal access and wider panoramic visualization of deep neural structures [70, 71].
It is important to note that these tumors frequently involve the cavernous sinus and internal carotid artery, and may extend intradurally toward the brainstem, hypothalamus, and internal capsule [52]. Careful resection of the tumor off of these elements is crucial in avoiding patient morbidity. Furthermore, chondrosarcomas have been shown to spread along the subperiosteal spaces and through the bone marrow. Therefore, if total resection is desired, extensive bone drilling should be performed along the tumor margins until bleeding marrow is reached [52]. Ultimately, the choice of surgical approach and extent of resection should be determined by the location of the tumor, the involvement of surrounding structures, the patient’s clinical status, and the operator’s experience and comfort with various techniques.
Radiation therapy has been shown to give a distinct advantage when performed in combination with surgery, regardless of the degree of resection [58, 62]. However, there is scarce comparison between the different types of radiation therapies available. Furthermore, most studies are limited in that they include both chordomas and chondrosarcomas, even though chondrosarcomas have been shown to be relatively more susceptible to radiation treatment [52, 63, 72, 73]. Radiation therapy as a primary treatment has been shown to be efficacious in certain situations; however, the outcomes are inferior to those tumors treated with both surgery and postoperative radiotherapy [58, 62]. As such, we suggest that all patients with chondrosarcomas of the skull base should be treated with surgical resection as the primary method of treatment followed by postoperative adjuvant radiation therapy.
Esthesioneuroblastomas
Esthesioneuroblastomas, also known as olfactory neuroblastomas, are rare, malignant neoplasms of neural crest origin that arise from the olfactory epithelium of the upper nasal cavity and anterior skull base [74–76]. These tumors may invade locally and spread to the paranasal sinuses, nasal cavity, and other surrounding structures, typically causing nasal obstruction and epistaxis (Fig. 39.3) [77, 78]. They may also extend superiorly across the cribriform plate intracranially to involve the brain or seed the cerebrospinal fluid. Metastasis may occur via the lymphatic or hematogenous route in 17–48 % patients, and is most commonly seen in the cervical lymph nodes and more distant sites, including the lungs, brain, and bone [79, 80]. Due to the rarity of these tumors and the heterogeneity of treatment modalities described in the literature, a standardized management protocol has not been established for esthesioneuroblastomas [80].


Fig. 39.3
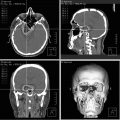
(a, b) Preoperative T1-weighted post-gadolinium MRI (a sagittal view, b coronal view) demonstrating an esthesioneuroblastoma with intracranial extension through the cribriform plate. An endoscopic endonasal transcribriform approach was performed for removal of the tumor with reconstruction of the resultant skull base defect. (c, d) Postoperative T1-weighted MRI (c sagittal, d coronal) shows gross total resection of the tumor. Packing is visualized in the nasal cavity bolstering the skull base repair
Craniofacial surgical resection has largely remained the preferred method of treatment and usually requires the removal of the cribriform plate in order to prevent perineural extension. Surgical resection is typically followed by adjuvant radiotherapy, and in some cases, chemotherapy in order to achieve better long-term results [80–84]. The decision by which the modality of therapy is chosen for treatment is largely driven by the characteristics of the tumor and primarily based on Kadish staging. According to most available studies, Kadish A (tumors limited to the nasal cavity) and Kadish B tumors (tumors that extend to paranasal sinuses) are optimally treated with craniofacial resection followed by postoperative radiotherapy for greater disease-free survival. Postoperative radiation therapy, in patients with Kadish A and B tumors, has shown to improve recurrence-free survival at 5 years when compared to patients treated with surgery alone [85]. Overall survival rates in patients treated with surgical resection followed by postoperative radiation therapy have been reported to be up to 100 % [86] and 5-year disease-free survival rates up to 85.7 % [87]. Recent literature also suggests that multimodality therapy with preoperative (neoadjuvant) chemotherapy followed by craniofacial resection and postoperative radiation therapy should be considered for Kadish C (tumors with skull base or intracranial extension) and higher-grade lesions (tumor with neck or distant metastases) [88, 89].
Over the past 2 decades, various advances in skull base techniques have been made to incorporate both endoscopic-assisted craniofacial resection and more recently, purely endoscopic endonasal resection of these tumors in addition to standard craniofacial surgical resection [90]. The introduction of endoscopy has transformed traditional open skull base surgery and confers various advantages over conventional craniofacial resection. These advantages include: (1) no retraction of the frontal lobes, (2) better visualization in difficult areas, (3) use of natural orifices (nasal corridor) to avoid visible incisional scar, (4) reduction in recovery time, and (5) less overall morbidity and mortality [91–95]. However, longer follow-up is necessary in order to better assess the recurrence-free survival rates and prognosis in patients with esthesioneuroblastoma resected via the purely endoscopic endonasal route. In our experience, careful patient selection is important when considering the purely endoscopic approach. We generally reserve the endoscopic approach for sinonasal tumors with minimal intracranial extension, and use a combined transbasal bifrontal approach and endoscopic endonasal approach (combined cranionasal) for sinonasal tumors that have more extensive intracranial involvement. Additionally, the role of SRS in management of esthesioneuroblastomas has not been very well defined and further studies are required with longer follow-up in order to assess whether this treatment modality is efficacious [86, 96–98].
Craniopharyngiomas
Craniopharyngiomas are benign, epithelial-squamous, extra-axial tumors that arise along the path of the craniopharyngeal duct or Rathke’s pouch, and are designated as WHO Grade 1 tumors [99, 100]. They have an overall incidence of 0.5–2 per 100,000 [101] and represent approximately 5 % of all intracranial tumors and 5–10 % of all pediatric intracranial tumors (Fig. 39.4) [102, 103]. These tumors typically exhibit a bimodal distribution, with peak incidences at 5–14 years and 50–74 years [101, 104], with childhood craniopharyngiomas accounting for 30–50 % of all cases [101, 105]. They are classified into two histopathological subtypes, adamantinomatous craniopharyngiomas, which are associated with a mutation of the β-catenin gene and predominantly occur in the first 2 decades, and papillary craniopharyngiomas, which typically occur in adults [106]. Some authors have suggested lower recurrence rates in those with papillary histology, but others have reported no difference in outcome or recurrence between the two subtypes [106, 107]. Grossly, they are typically characterized as cystic, solid, and mixed, and many tumors exhibit extensive calcification.


Fig. 39.4
(a, b) Preoperative T1-weighted post-gadolinium-enhanced MRI (a sagittal, b coronal) demonstrating a pediatric craniopharyngioma resulting in visual loss with optic compression. An endoscopic endonasal transplanum transtuberculum approach was performed. (c, d) Postoperative T1-weighted post-gadolinium-enhanced MR images (c sagittal, d coronal) show gross total resection of the tumor with decompression of the optic apparatus
While these tumors are classified as benign, they can sometimes exhibit tumor adherence to critical structures in the parasellar region, including the optic apparatus, pituitary stalk, pituitary gland, hypothalamus, third ventricle, anterior cerebral artery complex, and surrounding perforating arteries. Hypothalamic involvement is of particular concern, as it has been shown to be significantly related to pre- and postoperative morbidity, including behavioral disturbances, obesity, cognitive decline, and an overall decrease in quality of life [108–110]. Because of this, a separate classification system has been developed based on and the degree of hypothalamic involvement, with Grade 0 tumors having no involvement, Grade 1 exhibiting compression of the hypothalamus, and Grade 2 involving the hypothalamus. Furthermore, the clinical presentation of craniopharyngiomas varies by the anatomical location of the lesion with relation to the optic chiasm. Prechiasmatic lesions typically involve the optic apparatus, resulting in decreased visual acuity and visual field defects, usually presenting as bitemporal hemianopsia in up to 49 % of cases [100, 111, 112]. Retrochiasmatic lesions commonly present with hydrocephalus and signs of increased intracranial pressure, including headache, nausea, vomiting, papilledema, and horizontal double vision [100, 113]. Intrasellar lesions typically present with headache and endocrinopathies, including polyuria, polydipsia, growth retardation, developmental disturbances during puberty in children, hypogonadism in adults, and significant weight gain [100, 114]. However, despite these trends associated with anatomical location, many patients will present with a combination of these clinical features.
Obtaining GTR has traditionally been the primary goal of surgical management due to the relatively high rates of recurrence. It has been shown that tumor recurrence is present in approximately 10–30 % of cases treated by GTR and up to 50 % in cases treated without GTR [104, 106, 109, 111, 112, 115–118]. Furthermore, recurrent tumors are associated with worse morbidity, mortality, and decreased long-term survival. However, this paradigm is currently being put into question, especially in cases where GTR might result in significant morbidity [109, 110]. Some authors suggest that significant hypothalamic involvement (i.e., Grade 2) is a strong indicator to undergo subtotal resection followed by radiation therapy as opposed to GTR [110, 117, 119]. Additionally, some recommend minimal resection, such as cyst decompression followed by radiation therapy in elderly patients with multiple comorbidities, or in patients with stalk infiltration but an intact hypothalamic–pituitary axis [117]. Nevertheless, a GTR should be attempted whenever safely possible.
Initially, transcranial routes, including pterional, orbitozygomatic, subfrontal, transcallosal, and bifrontal approaches, were the method of choice for accessing these lesions [103, 104, 106, 109, 111, 112, 115, 116, 119–121]. While these approaches can expose a wide variety of anatomical locations, they typically require significant brain retraction and manipulation of neurovascular structures in order to obtain optimal visualization and surgical resection [117]. The extended transsphenoidal microsurgical approach, utilizing an operative microscope through a nasal speculum, provides a more direct route to the sellar and suprasellar regions [103, 117, 122, 123]. Recently, endoscopic endonasal approach (transplanum transtuberculum) has emerged as a viable treatment option for resection of these tumors [117, 124]. The use of an endoscope has enabled surgeons to obtain a wider field of view, allowing minimally invasive resection of these tumors with minimal manipulation of surrounding structures. In general, studies have shown similar rates of GTR among the different approaches, ranging from 9 to 90 % for transcranial approaches, 12–90 % for microscopic transsphenoidal approaches, and 29–86 % for endoscopic endonasal transsphenoidal approaches [104, 106, 109, 111, 112, 115–117, 119, 120, 122, 123]. Ultimately, the decision of which operative approach to use largely depends on the specific goals of the patient as well as the training and technical preference of the neurosurgeon.
As previously mentioned, radiation treatment has become a commonly used therapy in certain cases of craniopharyngiomas. Rates of tumor control and mortality have been shown to be comparable in cases where subtotal resection with radiation therapy was chosen over GTR or when radiation therapy was chosen for the treatment of recurrent tumors [118, 125, 126]. However, studies addressing the use of SRS are currently very limited. Currently, SRS is used for small, solid craniopharyngiomas located 3–5 mm away from the optic nerve or chiasm [127] or in cases of recurrent or residual tumors following subtotal or GTR [128]. In general, surgical resection remains the treatment of choice for craniopharyngiomas, with GTR being the ultimate goal when obtainable. However, more studies are needed to compare the long-term outcomes of various combinations of surgical techniques and radiotherapy interventions in order to establish the best possible course for patient care.
Skull Base Meningiomas
Skull base meningiomas are typically difficult lesions to resect because of their location and close proximity to vital neurovascular structures. These tumors include, but are not limited to, orbital, optic nerve sheath, olfactory groove, tuberculum sellae, cavernous sinus, sphenoid wing, cerebellopontine angle, petroclival, and tentorial meningiomas. The other inherent difficulty in treating these lesions is that skull base meningiomas tend to differ in volume, shape, location, and clinical symptomatology. Optimal management of these tumors involves GTR with removal of the invaded and hyperostotic bone and surrounding dura, if safely possible [129, 130]. In many cases, only subtotal resection can be achieved, and in these cases postoperative radiosurgery or radiotherapy is often required for tumor control [131].
Over the past 2 decades, it has been well documented that extent of resection of skull base meningiomas correlates with long-term tumor recurrence [132–136]. Therefore, the key goals of the skull base meningioma treatment paradigm include: (1) maximal resection of tumor with minimal neurological deficit, (2) preservation of functional outcome, and (3) prevention of tumor recurrence [129]. Various approaches of microsurgical resection have been described for skull base meningiomas including infratemporal fossa [137], retrosigmoid [138], and extended approaches to the jugular foramen; the supraorbital [139], pterional [140], and orbitozygomatic [141] approaches for tuberculum sellae meningiomas; combined subtemporal-suboccipital approach, the preauricular-subtemporal-infratemporal approach [142], the posterior transpetrosal [143], anterior transpetrosal-transtentorial [144], and combined supratentorial and infratentorial approaches [145, 146] for sphenoid wing, petroclival, and tentorial meningiomas; the transoral approach to the lower clivus and upper cervical spine [43]; and the extreme lateral transcondylar approach [147–149] for ventral foramen magnum meningiomas at the craniocervical junction. More recently, endoscopic endonasal techniques have also been described and reported for certain midline skull base meningiomas such as planum sphenoidale, olfactory groove, tuberculum sellae, and clival meningiomas [150–157].
Among the most challenging meningiomas to treat surgically include cavernous sinus, tuberculum sellae, and petroclival meningiomas. Cavernous sinus meningiomas typically present with visual and ocular motor deficits such as ptosis, diplopia, anisocoria, and ophthalmoplegia, in addition to trigeminal nerve dysfunction [158]. Cavernous sinus meningiomas are inherently difficult tumors to resect because of their involvement of the cavernous sinus, internal carotid artery, and cranial nerves III–VI. The treatment strategies for cavernous sinus meningiomas have evolved over the past decade [159–163]. In many cases, subtotal resection and decompression of the cavernous sinus and optic nerve can be considered. In these instances, postoperative radiotherapy or SRS for residual tumor may be indicated if the meningioma displays atypical features on histology or if it is found to be malignant [129]. Long-term outcomes of Gamma Knife SRS for patients with small- or medium-sized cavernous sinus tumors have shown good results with respect to progression-free survival (90 and 75 %) and functional outcomes (93 and 91 %) at 5 and 10 years, respectively [164]. Tumor progression was more likely found to occur from margins that remain outside of the treatment volume [164]. Furthermore, tumors that are asymptomatic and radiographically stable over time are followed up at appropriate intervals without intervention.
Petroclival meningiomas (Fig. 39.5) are rare and make up only about 3–10 % of all posterior fossa meningiomas [165]. These tumors are particularly complex because of their deep location and often intimate relationship with vessels, nerves, and the brainstem. In addition, the natural history of these tumors may be unpredictable and frequently involves progressive growth and neurological deterioration [166, 167]. The neurologic presentation of petroclival meningiomas varies, though some of the more common symptoms include headache, gait disturbances, cranial nerve deficits, and other symptoms related to brainstem compression [166, 168]. Petroclival meningiomas are typically treated on a case-specific basis and numerous aspects of management are considered, including the size and location of tumor, choice of surgical approach, the postoperative evaluation of results, and treatment of recurrence [129, 138, 144, 145, 169–171]. For smaller tumors in symptomatic patients, GTR, if feasible, should be the goal of surgery. On the other hand, small and asymptomatic meningiomas, particularly in elderly patients, may be observed and followed up with serial neurological exams and imaging. Larger petroclival meningiomas may require combined surgical approaches to allow adequate tumor exposure and debulking [145, 146, 171]. In cases involving large or giant petroclival meningiomas, it is important to consider the patient’s postoperative quality of life [135]. In some cases, settling for a subtotal resection with decompression of adjacent structures is reasonable, with or without postoperative radiosurgery, particularly in cases that involve cavernous sinus invasion, pial invasion, and tumor adherence to cranial nerves and vessels [166].


Fig. 39.5
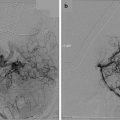
(a, b) Preoperative T1-weighted post-gadolinium MRI (a axial, b coronal) demonstrating a left petroclival meningioma with brainstem compression. The patient underwent a combined petrosal approach resulting in gross total resection of the tumor. (c, d) Postoperative T1-weighted post-gadolinium MRI (c axial, d coronal) shows complete removal of the tumor with brainstem decompression
Tuberculum sellae meningiomas make up 5–10 % of all intracranial meningiomas and pose a unique surgical challenge. They typically arise from the tuberculum sellae, chiasmatic sulcus, limbus sphenoidale, and diaphragma sellae [172]. These tumors commonly cause displacement of the optic chiasm and optic nerves and often have optic canal involvement. The most common clinical manifestation of tuberculum sellae meningiomas is progressive visual loss, classically known as “the chiasmal syndrome,” a term first coined by Harvey Cushing in 1930 to describe the hallmark presentation of these lesions [173]. The ideal treatment involves decompression of the optic apparatus by achieving a Simpson Grade I removal (i.e., surgical GTR of the tumor, its dural attachments, and associated hyperostotic bone) while preserving visual function, the integrity of critical adjacent neurovascular structures, and endocrine function [154, 173]. In cases where tumor extends into the optic canal, early bony decompression of the optic canal (including anterior clinoidectomy, optic canal unroofing, and incising falciform ligament) has been advocated during the removal of tuberculum sellae meningiomas and appears to have a strong influence on postoperative visual recovery [154, 173–177]. This decompression involves extradural, and in some cases, intradural removal of the anterior clinoid process as well as the unroofing of the optic canal followed by incision of the falciform ligament and dural sheath of the involved optic nerve. In addition to early decompression of the optic nerve, this particular maneuver allows exploration of the optic canal for additional tumor since this can be a frequent site of recurrence [154, 173, 174, 177–179].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree