Chapter Outline
Terms Used in Describing Small Bowel Obstruction and Adynamic Ileus
Classification of Small Bowel Obstruction
In patients with known or suspected small bowel obstruction, the radiologist is called on to answer the following questions:
- •
Is the bowel obstructed?
- •
If so, what is the level of the obstruction?
- •
What is the cause of the obstruction?
- •
What is the severity of obstruction?
- •
Does the patient have a closed loop or simple obstruction ( Fig. 46-1 )?

Figure 46-1
Simple versus closed loop small bowel obstruction.
In simple small bowel obstruction (Simple), there is a single discordance of small bowel caliber at the transition point (TP). There is tapering of the distention back to the ligament of Treitz (Lig of Treitz). In closed loop obstruction (Closed loop), the obstructed segment of bowel between the first transition point (TP A) and second transition point (TP B) becomes markedly dilated and is at risk for ischemia. There is tapering of the distention back to the ligament of Treitz proximal to TP A. The bowel is collapsed beyond the level of TP B. The degree of bowel caliber is triply discordant, most dilated between TP A and TP B, less dilated proximal to TP A, and collapsed beyond TP B. IC valve, ileocecal valve.
- •
Is the bowel strangulated?
- •
Are ischemic changes present?
- •
Is immediate surgery needed, or should a trial of conservative therapy be used?
- •
These questions are very important to answer because clinical findings may be misleading: approximately 88% of patients with partial small bowel obstruction resolve with conservative management within 48 hours; 37% of patients admitted with ischemic small bowel obstruction have a different clinical diagnosis. The preoperative diagnosis of strangulation is made on clinical grounds alone in 48% of patients. The purpose of this chapter is to provide a working classification for radiologic thinking about small bowel obstruction. After a description of the causes of small bowel obstruction, this chapter will present a radiologic approach to suspected small bowel obstruction ( Box 46-1 ).
Extrinsic Lesions
Adhesions
Hernias
External
Inguinal
Femoral
Obturator
Sciatic
Perineal
Supravesical
Spigelian
Lumbar
Incisional
Umbilical
Internal
Paraduodenal
Epiploic foramen
Diaphragmatic (traumatic)
Transomental
Transmesenteric
Iliac fossa
Extrinsic tumors in mesentery
Lymphoma
Peritoneal metastasis
Carcinoid
Desmoid
Abscess
Diverticulitis
Pelvic inflammatory disease
Crohn’s disease
Aneurysm
Hematoma
Endometriosis
Congenital
Annular pancreas
Ladd’s bands
Intramural Lesions
Tumors infiltrating wall of small intestine
Adenocarcinoma
Carcinoid tumor
Lymphoma (rare)
GI stromal tumors (rare)
Inflammatory conditions
Crohn’s disease
Tuberculosis
Potassium chloride or NSAID-induced stricture
Eosinophilic gastroenteritis
Vascular
Radiation enteropathy
Ischemia
Hematoma
Post-traumatic hematoma
Thrombocytopenia
Anticoagulants
Henoch-Schönlein purpura
Congenital
Atresia, stricture, stenosis
Duplication
Meckel’s diverticulum
Intraluminal Causes
Obturation
Gallstone
Bezoar
Foreign body
Ascaris
Meconium (cystic fibrosis)
Intussuscepting tumor
Inverted Meckel’s diverticulum
Terms Used in Describing Small Bowel Obstruction and Adynamic Ileus
Small bowel obstruction is a condition, not a disease. The term small bowel obstruction does not indicate the cause, severity, or prognosis of the obstruction. The term ileus comes from the Greek variably meaning “to twist up,” “to wrap,” or “to roll up,” implying that a patient is rolled up in discomfort. The term ileus means that the bowel lumen is dilated, but must have a qualifier before it that indicates neuromuscular-based dilation or mechanical obstruction. Functional ileus, paralytic ileus, functional obstruction, and adynamic ileus are equivalent terms indicating that the bowel is dilated because there is abnormal intestinal motility that prevents succus entericus from progressing down the gastrointestinal (GI) tract. Common causes of adynamic ileus include drugs that alter bowel motility (e.g., narcotics), recent abdominal surgery, intestinal ischemia, peritonitis, neuromuscular disorders (e.g., scleroderma), and endocrine disturbances (e.g., diabetes, hypothyroidism). If succus entericus cannot progress down the small bowel because the lumen is mechanically obstructed, the terms small bowel obstruction, mechanical ileus, or mechanical obstruction can be used. To avoid confusion, we will only use two distinct terms— adynamic ileus and small bowel obstruction .
Simple obstruction implies that the lumen is partially or completely occluded, but that blood flow is preserved. Strangulation or strangulated obstruction means that blood flow is compromised, leading to bowel wall edema, intestinal ischemia and, eventually, necrosis to perforation. A simple obstruction can be complete (i.e., no fluid or gas passes beyond the site of obstruction) or incomplete (i.e., some fluid and gas does pass beyond the site of obstruction). In open loop obstruction, intestinal flow is blocked distally, but the proximal loops are open and can be decompressed by vomiting or nasogastric intubation. In closed loop obstruction, both flow into and flow out of the closed loop are blocked, resulting in progressive accumulation of fluid in the closed loop (see Fig. 46-1 ).
Pathophysiology
After food has been digested in the stomach and duodenum, most of the contents entering the jejunum are in fluid state. If the neuromuscular function of the bowel is preserved, a large degree of luminal narrowing is needed to cause obstruction. Narrowing can be caused by abrupt angulation, kinking, or compression of the lumen by extrinsic disease; circumferential compromise of the lumen by intrinsic disease, intussusception, and/or obturation by intraluminal contents.
A series of physiologic factors leads to progressive accumulation of fluid in obstructed small bowel loops. Elevated intraluminal pressures stimulate secretion of water and electrolytes into the lumen. Release of hormones such as vasoactive intestinal polypeptide (VIP) and various prostaglandins stimulates epithelial secretion and inhibits fluid resorption. Enzymatic breakdown of intraluminal contents increases the osmolality of intraluminal contents, also drawing more fluid into the lumen.
Intraluminal pressures are higher with closed loop than with open loop obstruction, which leads to even further secretion of fluid into the closed loop. High intraluminal pressures eventually exceed venous pressures and then capillary pressures. Loss of blood flow leads to the potential cascade of edema, small bowel ischemia, necrosis, and perforation. Stasis in a closed loop obstruction also leads to bacterial overgrowth. Subsequent bacterial invasion of the bowel leads to further edema and inflammation. Release of bacterial toxins into the circulation causes shock.
In simple (open loop obstruction) of the proximal small intestine, vomiting or intubation leads to the loss of gastric, pancreatic, and biliary secretions. Loss of electrolytes in these secretions results in dehydration, metabolic alkalosis, hypochloremia, hypokalemia, and hyponatremia. Only minor changes in fluids and electrolytes are seen with open loop obstruction involving the distal small intestine because less fluid is lost.
Symptoms
Open loop (simple) obstruction of the proximal small bowel is characterized by frequent, large-volume, often bilious vomiting. Abdominal pain is intense, but is often relieved by vomiting. Abdominal distention and tenderness are minimal because the small bowel is decompressed by vomiting.
In open loop (simple) obstruction of the distal small bowel, fluid fills the distensible small bowel loops. As a result, vomiting is infrequent and of low volume. Abdominal distention is of moderate to marked degree.
In closed loop obstructions, reflex vomiting may be present, even if the obstruction is distal. Abdominal distention may be absent. Abdominal pain is progressive and may rapidly worsen if ischemia progresses to infarction and perforation.
Radiologic Imaging
This section discusses the role of a variety of imaging modalities in patients with small bowel obstruction.
Plain Radiography of the Abdomen
The plain radiograph of the abdomen has traditionally been used as the first radiologic study in the work-up of acute abdominal pain and suspected small bowel obstruction. Many adults admitted with clinical suspicion of acute small bowel obstruction undergo surgery on the basis of clinical, physical, and laboratory findings in conjunction with plain abdominal radiographs. The diagnosis of small bowel obstruction on abdominal radiographs relies on the demonstration of dilated loops of small bowel ( Fig. 46-2 ), identified by their smooth luminal contour, valvulae conniventes, and central location in the abdomen. Air-fluid levels may traverse the entire lumen loop of the loop) or be trapped as bubbles between folds at the top of a fluid-filled bowel loop, resulting in a string of pearls sign. Fluid-filled loops may not be seen on supine radiographs but may be recognized on erect or cross-table lateral decubitus radiographs.
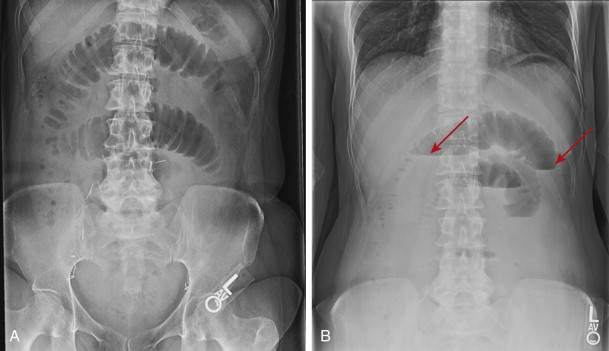
Plain abdominal radiographs have only moderate accuracy for the diagnosis of small bowel obstruction. In one series, abdominal radiographs had a sensitivity of 86% for the diagnosis of high grade obstruction, but a sensitivity of only 56% for the diagnosis of low-grade obstruction. In another series, abdominal radiographs revealed small bowel obstruction in 78% of patients with this condition. Obstructions can be missed by abdominal radiographs if vomiting or nasogastric intubation leads to decompression of small bowel loops. Fluid-filled loops ( Fig. 46-3 ) may not be visible, leading to a false-negative diagnosis or erroneous interpretation of the level of obstruction. In a few patients, abdominal radiographs may be obtained before fluid and air accumulates in the lumen and dilates the small intestine. A false-positive diagnosis of obstruction occurs in up to 44% of patients, mistaking adynamic ileus or aerophagia for obstruction. The level of obstruction can be accurately predicted in about 75% of patients.

Unfortunately, plain abdominal radiographs are poor at suggesting the diagnosis of strangulation. In patients with strangulating obstruction, the underlying small bowel obstruction is diagnosed in only 50% of patients. Strangulation is suggested on abdominal radiographs by the demonstration of smooth, thick valvulae conniventes in a dilated loop or loops of small intestine. Linear pneumatosis paralleling in the wall of the small bowel or portal venous gas strongly suggests superimposed ischemia and infarction. Closed loop obstructions are often not visible because they are fluid-filled. However, a dilated, fluid-filled loop that does not change location or configuration on serial abdominal radiographs suggests a closed loop obstruction.
Computed Tomography
Given the limitations of plain abdominal radiographs, computed tomography (CT) has become the premier imaging technique for the diagnosis of small bowel obstruction. CT has a vital role in these patients because it is readily available, fast, and offers a comprehensive, noninvasive means of assessing the bowel lumen, bowel wall, and adjacent mesentery and vascularity. In comparison to barium studies, CT does not rely on oral contrast reaching the site of obstruction, but uses intestinal fluid proximal to the obstruction to outline the transition zone (see Fig. 46-3 ). As a result, CT has become the imaging modality of choice for diagnosing a variety of conditions, including adynamic ileus, acute or prolonged high grade small bowel obstruction, suspected strangulation or perforation, and acute inflammatory processes such as appendicitis, Crohn’s disease, or diverticulitis causing small bowel obstruction. CT is also the best examination for evaluating patients with a known primary tumor and suspected metastatic disease causing small bowel obstruction ( Fig. 46-4 ).

CT has an accuracy of almost 90% for the diagnosis high-grade obstructions versus an accuracy of only 50% for low-grade obstructions. CT can accurately predict the cause of obstruction in 70% to 95% of patients and can often suggest superimposed ischemia and intestinal perforation. The entire abdomen is evaluated by CT, providing a vast array of information not demonstrated by an abdominal radiograph. For example, CT can identify a variety of causes of small bowel obstruction, such as carcinoid tumor, intraperitoneal or hematogenous metastasis, Crohn’s disease, appendicitis, and diverticulitis.
Although CT is the premier means of evaluating patients with small bowel obstruction, it does have limitations. It is difficult to distinguish an adynamic ileus of the small bowel from a distal small bowel obstruction if a transition zone is not identified. It can also be difficult to determine the location of the transition zone along the course of dilated small bowel, even with the ability to scroll through a scan on a picture archiving and communications system (PACS) workstation or visualize the intestine in any plane. The localization of the transition zone to an abdominal quadrant is not accurate in diagnosing the distance of the obstruction from the duodenal-jejunal junction because a markedly dilated jejunum may fall into the pelvis and an obstructed distal ileum may rotate into the left upper quadrant. Finally, although CT is an excellent technique for suggesting strangulation, it can usually but not always predict whether a bowel loop has reversible or irreversible ischemia or whether the affected small bowel will be viable at surgery. Abnormal bowel wall thickening or mesenteric engorgement also cannot always predict surgical viability of the bowel. In the setting of ischemia, a CT finding of pneumatosis indicates breakdown in the mucosal integrity of the bowel wall and is strongly suggestive of ischemia. The CT finding of pneumatosis can usually but not always predict irreversible ischemia at surgery. Patients with CT findings of free intraperitoneal gas or portomesenteric gas have a greater likelihood of irreversible transmural necrosis than patients with pneumatosis alone. Nevertheless, CT is currently the modality of choice for acutely ill patients with suspected high-grade or complete small bowel obstruction, suspected strangulation, or suspicion of an inflammatory process (e.g., appendicitis, Crohn’s disease) or a hernia as the cause of small bowel obstruction.
Barium Studies
Various barium studies may be performed on patients with suspected small bowel obstruction, including small bowel follow-through, enteroclysis (small bowel enema), barium enema, and studies performed via indwelling nasogastric, nasojejunal, or jejunostomy tubes.
The use of barium is safe in patients with suspected small bowel obstruction because barium does not form concretions in the small bowel lumen proximal to an obstructing lesion. In obstructed patients, the residual fluid in dilated small bowel loops prevents barium from dehydrating and agglomerating. Barium also does not form concretions in the proximal ascending colon. However, concretions may form in the remainder of the colon because water is resorbed from the barium sulfate suspension. Therefore, barium sulfate should not be administered orally or via a proximal bowel tube in a patient with an obstructing lesion distal to the hepatic flexure of the colon because dense barium concretions can form, with the possibility of stercoral ulceration and perforation. If there is any doubt about the location of bowel obstruction, CT or barium enema should be performed before barium is administered proximal to the site of a colonic obstruction.
The presence of barium proximal to a small bowel obstruction makes surgery more hazardous because barium that spills into the peritoneal cavity may result in a form of granulomatous peritonitis. Some surgeons therefore request that small bowel examinations be performed with water-soluble contrast material rather than barium. Small bowel follow-through examinations using water-soluble contrast have even been advocated as a therapeutic technique to improve small bowel obstruction.
The use of hypertonic water-soluble contrast in small bowel obstruction can be problematic. This draws fluid into the lumen, whereas the aim of therapy is to decompress the small bowel. Dilution of water-soluble contrast material by retained fluid also may result in very poor radiologic detail , compromising the ability to diagnose small bowel obstruction or demonstrate the site and cause of obstruction. These limitations are partially obviated by the use of iso-osmolar iodinated oral contrast agents.
Small bowel follow-through and enteroclysis studies are not recommended to be performed in patients with high-grade distal small bowel obstruction. Because of small bowel dilation and diminished small bowel peristalsis, it may take an inordinate period of time for barium to reach the site of obstruction. Not infrequently, 24-hour follow-up radiographs must be obtained to visualize the site of a high-grade distal small bowel obstruction. By this time, the barium suspension is diluted and there is poor anatomic detail of the transition zone ( Fig. 46-5 ). The transition zone may even be obscured by dilated-barium filled loops.

If a study must be performed from above in a patient with a high-grade small bowel obstruction, prior passage of a Miller-Abbot tube, Cantor tube, or enteroclysis catheter with a built-in suction port is helpful. The small bowel is first decompressed and then an antegrade barium study is performed.
If more information is needed after CT, a single-contrast barium enema with reflux of barium to the site of small bowel obstruction is often better than an antegrade study in patients with high-grade distal small bowel obstruction ( Fig. 46-6 ). After the administration of 1 mg of intravenous (IV) glucagon to relax the ileocecal valve, barium can be refluxed into the distal ileum in 85% of patients. Barium is then pushed retrogradely by the addition of water to the enema bag. This is an excellent technique that can easily visualize pelvic loops of ileum and even the proximal ileum or distal jejunum. A barium enema is also an excellent technique in patients with an ileocolic anastomosis. A long length of small intestine can be visualized by retrograde flow of barium through the ileocolic anastomosis, resulting in the excellent demonstration of a transition zone. In patients with an ileostomy or colostomy, a retrograde ileostomy or colostomy study can also be performed to diagnose a distal small bowel obstruction.

Enteroclysis and small bowel follow-through examinations are reserved for patients with known or suspected low-grade small bowel obstruction. Enteroclysis should not be performed in patients with high-grade small bowel obstruction, suspected strangulation, suspected perforation, or adynamic ileus. Enteroclysis is superior to small bowel follow-through for the demonstration of short lesions such as strictures or tumors. By overdistending the small bowel lumen, enteroclysis can demonstrate subtle adhesions. Obstruction is implied by distention of the jejunal diameter to more than 4 cm and ileal diameter to more than 3 cm. A high-grade obstruction is implied by delayed passage of contrast to the transition zone, dilution of contrast material, and minimal passage of contrast material through the transition zone. A low-grade obstruction is diagnosed if there is delayed passage of contrast at the transition zone itself. Small bowel follow-through examinations are more physiologic, however, with inherent small bowel motility propelling the barium down the intestinal tract, allowing a more accurate estimate of transit time. Small bowel follow-through examinations are also much easier on the patient and examiner, but only in the setting of low-grade or absent small bowel obstruction. Long lesions such as Crohn’s disease and ischemia ( Fig. 46-7 ) can be easily demonstrated on small bowel follow-through in patients with low-grade obstruction. Small bowel follow-through examinations (especially with a peroral pneumocolon) are probably superior for the demonstration of the terminal ileum in comparison to enteroclysis.

Other Studies
CT and magnetic resonance (MR) enteroclysis are intubation techniques discussed in earlier chapters. Similar to enteroclysis, these techniques have no role in patients with suspected high-grade small bowel obstruction. These techniques may be of value for patients with carcinoid tumor, radiation therapy, and peritoneal metastasis. Transabdominal ultrasound and conventional MR imaging should be considered as primary imaging modalities for evaluating bowel obstruction only in the pediatric and pregnant population.
Classification of Small Bowel Obstruction
Extrinsic Lesions
Adhesions
Adhesions are the most common cause of small bowel obstruction, accounting for about two thirds of cases. Most adhesions form after abdominal surgery; 10% to 15% of adhesions are attributed to prior or concurrent inflammation. A small fraction of adhesions are thought to be congenital in origin. Although adhesions form in more than 90% of patients who have undergone laparotomy, only 5% of patients who have undergone laparotomy will develop an adhesive obstruction.
About 1% of patients will develop a small bowel obstruction in the immediate postoperative period. About 90% of these early postoperative obstructions are caused by adhesions ( Fig. 46-8 ) and 7% by hernias, with the remainder related to abscess formation, intussusception, or technical factors.

The most important radiographic finding in the diagnosis of small bowel obstruction is the abrupt transition from a dilated to nondilated small intestine at the site of obstruction. On barium studies, a sharp, straight, or curved edge ( Fig. 46-9 ) is seen where the adhesive band crosses the bowel lumen. The adhesion itself may be manifest as a narrow, bandlike radiolucency crossing the bowel lumen between dilated proximal and collapsed distal loops. The bowel may be angulated toward the adhesion. Smooth, thin mucosal folds will be tethered toward the extraluminal adhesive process. The bowel may be completely cut off, with a rounded, beaklike, or sharp edge. Mucosal folds are preserved and no mucosal nodularity or mass is present. Several loops may be pulled together at the site of adhesions. Multiple bands or extensive adhesions may be present. Multiple bands are often associated with scar formation along the anterior abdominal wall at prior incision sites. Extensive adhesions are often caused by prior extensive intra-abdominal inflammatory processes, such as traumatic perforation, peritonitis, and postoperative abscesses.

Adhesions are not usually seen on CT. The CT diagnosis of adhesions is a diagnosis of exclusion: there is an abrupt transition from a dilated to collapsed small intestine without apparent cause ( Fig. 46-10 ). The transition zone may appear rounded or beaklike. Other abnormalities can cause a transition zone without apparent cause on CT, including small primary tumors, such as carcinoid tumor, small intraperitoneal metastases, and short inflammatory, ischemic, or drug-related strictures. The CT diagnosis of adhesion is supported by a history of prior laparotomy in the absence of a history of a tumor with a predilection for peritoneal spread (e.g., ovarian carcinoma) or a known history of inflammatory bowel disease. The CT diagnosis of adhesions is accurate in 70% to 95% of patients.

Closed Loop Obstruction and Strangulation
The most common causes of closed loop obstruction are single adhesive bands, internal or external hernias, or rents in the mesentery. The trapped loop or loops become progressively dilated and fluid-filled. The vessels feeding the trapped intestine may be compressed by the band or hernia orifice or occluded by twisting of the mesentery; either process results in strangulation. Both dilation and the risk of strangulation of the trapped loops depend on the stage and degree of compression or twisting.
The radiographic findings of strangulation vary, depending on the level of trapping and twisting. Initially, the closed loop may not be dilated. In the usual patient, however, the closed loop is dilated and fluid-filled, with little or no intraluminal gas. If the closed loop(s) are parallel to the plane of reconstruction on CT, they appear as a C- or U-shaped configuration. If the loops are imaged perpendicular to the plane of reconstruction, they appear in a radial configuration, with the trapped mesentery pointing toward the band or hernia orifice ( Fig. 46-11 ). The loops that enter and exit the closed loop lay side by side and are narrowed or tapered at the level of the band or mesenteric rent. The entry or exit site may have a beaklike appearance when imaged in cross section. The small intestine proximal to the closed loop may be dilated and fluid-filled, whereas the small intestine distal to the closed loop is collapsed. The mesenteric vessels radiate to the point of obstruction.











