SPECT and PET in Benign Thyroid and Parathyroid Disease
Ujwal N. Bhure
Daniela B. Husarik
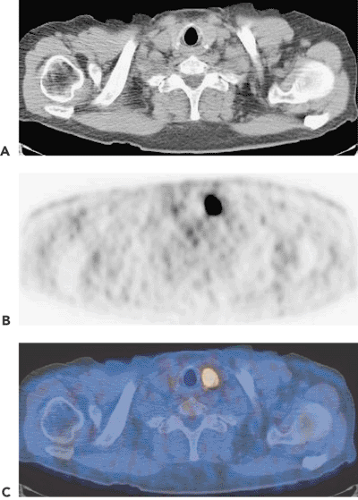
The role of integrated single-photon computed tomography (SPECT) and integrated positron emission tomography and computed tomography (PET-CT) in imaging thyroid and parathyroid disease is still largely undefined. SPECT and SPECT-CT in thyroid disease may play a role in dosimetry prior to radioiodine therapy, but dosimetry is more relevant in malignant thyroid disease, which is treated in Chapter 37. While PET and PET-CT have no role in thyroid disease per se, incidental fluorodeoxyglucose (FDG) uptake is occasionally noted on PET and PET-CT scans performed for oncology staging. Focal disease represents a malignancy in almost 50% of cases (see Fig. 63.1) and therefore requires invasive diagnosis, while diffuse disease indicates thyroiditis, which has to be worked up according to standard clinical practice.
Parathyroid imaging with SPECT and SPECT-CT is a relevant imaging modality, particularly in the identification of parathyroid adenomas in recurrent parathyroid disease. SPECT provides better localization and better differentiation from thyroid tissue and therefore is evolving into a standard imaging procedure. Whether the anatomic information provided by SPECT-CT adds clinical value has not yet been determined. There have been reports that imaging performed with carbon 11 [11C]methionine is useful for identifying parathyroid adenomas and may be superior to standard radionuclide imaging. Unfortunately, this seems not to be the case with fluorine 18 [18F]ethyl tyrosine, another PET amino acid uptake and metabolism tracer.
Introduction
The thyroid gland normally is located in the anterior lower neck. Substernal extension into the superior mediastinum may occur. Nuclear scintigraphy provides functional information about the gland, whereas cross-sectional imaging, including ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI), provide predominantly anatomic information. The commonly used radiotracers for scintigraphic studies are technetium 99m [99mTc]pertechnetate, iodine 123 (123I), and iodine 131 (131I) (1,2). Planar thyroid scintigraphy is a very commonly used technique in nuclear medicine in the workup of thyroid disease, while single-photon emission computed tomography (SPECT) is utilized infrequently for this purpose. The application of thyroid scintigraphy SPECT is mainly restricted to thyroid volume estimation.
Thyroid Volume Estimation with SPECT and SPECT-CT
Thyroid volumetry is used for dose estimations for thyroid therapy. Although morphological methods permit excellent volumetry of anatomic volume, thyroid therapy with
radioiodine requires functioning iodine volume. Hence, there is some value in using SPECT or SPECT-CT for this purpose.
radioiodine requires functioning iodine volume. Hence, there is some value in using SPECT or SPECT-CT for this purpose.
SPECT as planar scintigraphy typically uses a single intravenous administration of 120 MBq of [99mTc]pertechnetate. With the patient in supine position, a 180 degree anterior rotation (starting from the 270 degree position) is completed, with 32 azimuths at 25 seconds per azimuth. It is advantageous to use transmission correction either with transmission sources such as gadolinium 153 (153Gd) transmission line sources or CT when a SPECT-CT camera is used. Transmission scans are reconstructed using filtered back-projection and are corrected for downscatter of 99mTc into the 153Gd window. For the emission scan, an iterative maximum likelihood reconstruction algorithm with attenuation correction and window-based scatter correction as well as resolution recovery is typically used. For noise reduction, a 3-D edge-preserving 3 × 3 × 3–point median filter is applied (3). Various algorithms for identifying the thyroid volume on SPECT have been utilized. One established method is to use the largest transaxial cross-section of the thyroid and then draw a region of interest (ROI) of 4 × 4 pixels in the center of the larger thyroid lobe. If both lobes appear equally large, the right lobe is chosen. Within this ROI of maximum activity, the average pixel value is calculated. A threshold of 45% of this value is used for the segmentation of the thyroid gland. This threshold has been established in patient studies using a minimal mean squared error method (3).
A similar procedure can be used with SPECT-CT, but the anatomic information provided by the CT data will allow for much more precise edge detection. Because iodine dose is dependent on many factors besides thyroid uptake, the additional precision of volumetry of functioning thyroid tissue may not be relevant clinically for dose determination, and so the additional effort expended on dosimetry with this method in benign disease is mainly of scientific interest, while in malignant disease the clinical relevance may be higher (see Chapter 37).
Thyroid PET
Although a benign disease of the thyroid is not an indication for PET per se, FDG partial- or whole-body PET performed for tumor staging in other diseases does sometimes detect incidental thyroid lesions. Metabolic activity detected on PET is not, however, specific for malignancy but also demonstrates infectious and inflammatory disorders (4). The mechanism of FDG uptake in the thyroid gland is not known. Lymphocytic infiltration is a histologic characteristic of chronic thyroiditis. It has been reported in conditions with activated lymphoid tissue, such as sarcoidosis, Warthin tumor, and reactive lymphadenitis, and the same mechanism may be at work in chronic thyroiditis (5,6). The identification of such diffuse uptake should prompt further assessment of the thyroid. The growing number of PET examinations will likely lead to detection of an increased number of incidental thyroid lesions in patients without clinical symptoms. Concurrent with the etiology of thyroid disease, the FDG uptake may be focal or diffuse.
Focal FDG Uptake in the Thyroid
Incidental thyroid nodules identified in routine FDG-PET for benign or malignant disease were reported to be present in about 2.3% of 4,525 PET studies of patients with no prior history of thyroid disease. Biopsy of 15 of the 102 positive patients revealed thyroid cancer in 7 patients (47%), and 6 patients (40%) had nodular hyperplasia (Fig. 63.1) (7). Consequently, such an incidental finding requires further workup. Gain-of-function mutations of the thyrotropin receptor (TSHR) gene have been invoked as one of the major causes of toxic thyroid adenomas. 18F-FDG uptake was higher in these cases. Focal autonomous nodules are associated with focally enhanced glucose metabolism. Disseminated autonomous goiters show various patterns of focal or global hypermetabolism. Autonomous thyroid tissue caused by constitutive mutations of the TSH receptor is characterized by simultaneous increases in glucose and iodine metabolism (8).
Hence, the differential diagnoses for focal disease include a thyroid malignancy, a metastasis to the thyroid, and a benign nodular thyroid condition. Diagnosis can typically be made with fine needle aspiration.
Diffuse FDG Uptake in the Thyroid
While focal disease is more likely to be tumorous, diffuse disease is more suggestive of an inflammatory thyroid affection. Diffuse FDG uptake can be an indicator of thyroiditis such as Hashimoto or de Quervains thyroiditis. Obviously, because of its painless nature and the frequent initial lack of significant other symptoms, Hashimoto thyroiditis is more likely to be incidentally diagnosed by FDG-PET. FDG-PET is also positive in Graves disease and has been used to measure glucose metabolism in thyroid glands thus affected. FDG uptake was seen to be significantly higher in Graves disease patients than in controls. In these patients, FDG uptake increased with increasing antithyroid antibodies and shorter radioiodine half-life. Glucose metabolism is enhanced in the thyroid of Graves disease patients due not only to enhanced fractional blood volume but also to enhanced utilization. Whether lymphocytes present or thyroid epithelial cells utilize this surplus of glucose cannot be determined using in vivo PET measurements in humans. Still, the correlation of radioiodine half-life and glucose hypermetabolism suggests direct or indirect connections between glucose metabolism and hormone synthesis in thyroid cells (9).
Symmetrically increased uptake of FDG in the skeletal muscles and thymus is a clue for the diagnosis of Graves disease. The uptake of FDG in skeletal muscles is more specific in the psoas and rectus abdominis muscles. An increment of muscle FDG uptake may be responsible for the high peripheral glucose utilization seen in Graves disease (10). The enhancement of metabolic rate by thyroid hormone in target tissues is accompanied by increased glucose utilization, which in skeletal muscle and many other tissues is regulated at the level of membrane transport. Thyroid hormone may be an important regulator of the muscle/fat glucose transporter in skeletal muscle (11).
SPECT and SPECT-CT in Parathyroid Disease
Primary hyperparathyroidism (PHP) is a condition characterized by the overproduction of parathyroid hormone. It is a common endocrine disorder that occurs in about 1 in every 500 women and 1 in every 2,000 men over the age of 40 years (14). In the majority of patients (85%), a solitary adenoma is the underlying cause (15). Multiple adenomas and parathyroid hyperplasia are much less common, present in 4% and 10% of patients, respectively (16). Other rare causes include parathyroid carcinoma and parathyroid cysts. Only about 1% of cases of hyperparathyroidism are related to parathyroid carcinoma (17,18). There is general consensus that surgical excision is the treatment of choice for most cases of symptomatic and asymptomatic primary hyperparathyroidism, with a reported success rate of 90% to 95% (19). For successful surgery, exact localization of the lesion is considered to be increasingly important, particularly with advances in minimally invasive surgery, where accurate preoperative localization
of the hyperfunctioning parathyroid tissue is essential for successful surgical treatment. The onus of identifying this hyperfunctioning parathyroid tissue falls on imaging techniques such as high-resolution ultrasound, radionuclide imaging, CT, and MRI. Radionuclide scintigraphy has proved to be a reasonably accurate method for locating parathyroid adenoma or hyperplasia (20), but the choice of method depends on the patient’s state. As all imaging modalities have a relatively low sensitivity for the detection of parathyroid adenomas (21), the major use of imaging is still in cases recurrent disease, and many advocate using only ultrasonography when a patient presents with the first manifestation of hyperparathyroidism. Scintigraphic methods are unequivocally advocated in recurrent hyperparathyroidism for two reasons: there is a higher complication rate in reoperation, demanding accurate preoperative localization, and morphological imaging is compromised by scarring (22).
of the hyperfunctioning parathyroid tissue is essential for successful surgical treatment. The onus of identifying this hyperfunctioning parathyroid tissue falls on imaging techniques such as high-resolution ultrasound, radionuclide imaging, CT, and MRI. Radionuclide scintigraphy has proved to be a reasonably accurate method for locating parathyroid adenoma or hyperplasia (20), but the choice of method depends on the patient’s state. As all imaging modalities have a relatively low sensitivity for the detection of parathyroid adenomas (21), the major use of imaging is still in cases recurrent disease, and many advocate using only ultrasonography when a patient presents with the first manifestation of hyperparathyroidism. Scintigraphic methods are unequivocally advocated in recurrent hyperparathyroidism for two reasons: there is a higher complication rate in reoperation, demanding accurate preoperative localization, and morphological imaging is compromised by scarring (22).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree