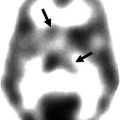
123I-FP-CT SPECT
123I-IBZM SPECT
Primary indications
Primary indication
Differentiating essential tremor from parkinsonian syndromes related to PD, MSA and PSP
Differential diagnosis of parkinsonian syndromes
Differentiation of dementia with LBD from other dementias
Secondary indications
Secondary indications
Diagnosis of early PD
Assessment of D2 receptor blockade during therapy
Evaluation of disease severity
Huntington’s disease
Differentiating presynaptic Parkinsonism from other form
Wilson’s disease severity of neurological symptoms
Pituitary adenoma
In a multicentre stage III trial in 160 patients suffering a parkinsonian syndrome (PS) versus 29 patients with essential tremor (ET) and 35 healthy volunteers, the ability of DaTscan to differentiate between PS and ET was assessed. An intention to treat and institutional read analysis demonstrated a sensitivity of 97.5 % and a 100 % specificity. It was concluded that DaTscan is able to differentiate between parkinsonian syndromes and essential tremor with an accuracy of 97 % (Benamer et al. 2000).
In the studies of Booij et al. and Marek et al., the correlation between imaging findings and disease severity was assessed. It should be stressed that a better correlation existed between decreased uptake and bradykinesia or rigidity as compared to tremor. A good correlation was found between striatal uptake ratios and disease duration, with an annual loss rate of 6–13 % in IPD versus 0–2.5 % in controls (Booij et al. 1997, 1999; Marek et al. 2001).
A procedure guideline by the EANM in 2002 already supported legitimacy of the use of 123I-labelled dopamine transporter ligands for the following ‘common indications’: confirmation or exclusion of dopamine nerve cell loss in parkinsonian syndromes. DaT-SPECT was considered valuable to discriminate essential tremor and DOPA-responsive dystonia from parkinsonian syndromes [e.g. PD, MSA or PSP]. Further indications included the evaluation of inconclusive cases to confirm or exclude presynaptic dopaminergic nerve cell loss, establishment of early diagnosis as DaT-SPECT imaging is suitable to assess the presynaptic deficit in early PD, assessment of severity of disease as DaT binding is related to the clinical stage and severity of PD and last but not least, measuring disease progression as in PD and non-PD patients that the progressive loss of DaT binding can be monitored over time with DaT-SPECT (Tatsch et al. 2002).
A meta-analysis in 2007 by Vlaar et al. concluded that a presynaptic radiotracer is relatively accurate in the differentiation between (early phase) PD and normal aging, patients with PD and those with ET or patients with PD and vascular Parkinsonism. The SPECT data was compared with clinical follow-up (Vlaar et al. 2007).
27.2.2 Tracers and Nuclear Imaging Protocols
Nuclear imaging is based on the visualization of (patho)physiological processes that underlie PD and other degenerative movement disorders. In order to better appreciate the underlying imaging technique, presynaptic pathways will be shortly discussed.
Vesicles within the presynaptic neuron store dopamine by the action of the vesicular monoamine transporter-2 (VMAT-2). On activation of the neuron, these vesicles release their dopamine content into the synaptic space. Reuptake of dopamine from the synaptic space relies on the dopamine transporter, which is located in the membrane of the presynaptic nigrostriatal nerve terminals.
To evaluate the presynaptic dopaminergic system using SPECT, the reuptake of dopamine by the dopamine transporter is targeted. There are a variety of 123I-labelled cocaine analogues that bind with high affinity to the dopamine transporter and are suitable for SPECT imaging (Halldin et al. 2001). Presently β-CIT (DOPA-scan) and FP-CIT (DaT scan) are commercially available and predominantly used; therefore, tracer preparation and image acquisition are further discussed.
Procedure
It is suggested that patients should avoid taking any medication or drugs of abuse that may influence visual and quantitative assessment of dopamine transporter scans (Darcourt et al. 2009). Therefore, a medication-free interval of five times the biological half-life is advised. Whether or not patients did adhere to this rule should be checked before injecting the radiopharmaceutical. If medication was not adequately stopped, postponing the investigation is strongly advised.
At least 5 min before tracer injection, the thyroid gland may be blocked by at least 200 mg sodium perchlorate as to prevent excessive free iodine uptake within the thyroid (information leaflet GE Healthcare ®). The tracers themselves are injected intravenously with a typical dose of 150–250 MBq. The time from injection to start of data acquisition is 18–24 h for β-CIT and 3–6 h for FP-CIT (Darcourt et al. 2009). The information leaflet provided by GE Healthcare ® states that adverse reactions occurred in less than 1 % of a group of 942 subjects. These consisted of headache, nausea, vertigo, dry mouth or dizziness.
Data Acquisition
For optimal SPECT imaging, it is important to position the detectors as close as possible to the head of the patient (<13 cm). LEHR or LEUHR collimators on a double-headed gamma camera are preferably used. During acquisition the camera heads rotate 180° (64 views) around the head of the patient. After acquisition, data are reconstructed using filtered back projection (FBP) or iterative reconstruction. If possible, attenuation correction is strongly recommended, using either a calculated homogeneous correction matrix according to Chang or a CT acquired attenuation map (Darcourt et al. 2009).
Interpretation and Quantification
In addition to visual assessment of images, semiquantitative analysis is strongly recommended as it may lead to more accurate assessment of images (Darcourt et al. 2009).
In both cases, symmetry of striatal DaT binding is assessed and reported. Semiquantitation may also be used in a follow-up setting in case of suspected disease progression or for monitoring of therapy effectiveness.
Quantification is done by drawing a region of interest (ROI) around the striatum and striatal subregions (caudate nucleus, putamen). The ROIs are drawn to calculate specific binding values:
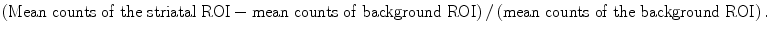
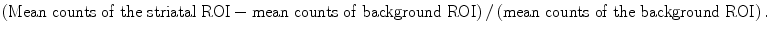
As reference, region-specific regions with known low or absent dopamine transporter uptake are chosen as to assess the amount of nonspecific binding. These regions may consist of occipital cortex and cerebellum. ROIs are drawn using visual contouring, threshold methods or using templates or overlay with an MRI scan. The latter is specifically useful in cases with already visual low to absent dopamine transporter uptake in the striatal region (Darcourt et al. 2009).
The calculated specific binding of striatum and striatal subregions may be compared to the specific binding values of an age-matched normal control group. A significant (linear) decline of 4–7 % per decade with age has been reported (Varrone et al. 2013). However, while Booij et al. also found a significant age-associated decline of [I-123]FP-CIT binding to striatal dopamine transporters in controls, they did not so in parkinsonian patients. This finding might give further support for the existence of an age-independent threshold in PD (Booij et al. 2001). Finally, while gender differences related to oestrogen levels have been noted in PD, we are not aware of databases taking into account these differences in clinical evaluation (Haaxma et al. 2007).
27.2.3 SPECT Versus PET
The current clinic of nuclear medicine comprises both SPECT and PET investigations. The PET counterpart of DaTscan is an 18F-DOPA PET scan. Before comparing both scan types, the general principle, preparation and image acquisition of FDOPA PET will be discussed.
18F-FDOPA PET Imaging
PET scans using 6-[18F]-fluoro-L-3,4-dihydroxyphenylalanine (18F-FDOPA) allow quantification of striatal DOPA decarboxylase activity and storage capacity of 18F-dopamine.
The use of catechol-O-methyltransferase (COMT) inhibitors should be ceased before the PET scan. It is however highly recommended to let the patient fast for at least 24 h, with particular attention for reducing amino acid uptake. Prior to the intravenous injection of 100–200 MBq 18F-FDOPA, the patient receives 2 mg/kg carbidopa (a peripheral AADC inhibitor), with a maximum of 150 mg, in order to increase plasma availability of the tracer to organs other than the pancreas. The acquisition consists of a 3D static imaging after 90 min, with 6 min acquisition time. Images are corrected for attenuation similar to SPECT investigations. The data analysis is both visually and quantitatively, in which case ROIs are drawn around striatum and striatal subregions (Eidelberg et al. 1990; Ishikawa et al. 1996; Eshuis et al. 2006).
Comparing the Two Modalities
The major differences between DaT-SPECT and 18F-FDOPA PET are listed in Table 27.2.
Table 27.2
Parameters of the DaTscan and 18F-FDOPA PET scan
DaTscan | 18F-FDOPA PET | |
|---|---|---|
Radionuclide | 123I, γ-rays, cyclotron produced | 18F, β-rays, cyclotron produced |
Drug-free interval | Yes for cocaine derivatives, in general PD medication may be continued | No |
Necessity of fasting | No | Yes |
Acquisition time | 45 min | 6 min |
Effective dose | Ca. 5 mSv | Ca. 4 mSv |
Age effect | Yes | No |
First of all, it has been suggested that loss of dopamine transporters in PD may be more severe than loss of dopaminergic neurons and thus may cause an increase in SPECT sensitivity (Lavalaye et al. 2000; van Dyck et al. 2006). Whereas the plasma membrane DA transporter is downregulated in the striatum of patients with PD, a study by Lee et al. suggested that the activity of aromatic L-amino acid decarboxylase is upregulated (Lee et al. 2000).
Two clinical studies, however, showed a good correlation between the specific binding values of FP-CIT and 18F-DOPA PET in patients with known PD (Ishikawa et al. 1996; Eshuis et al. 2006). Eshuis et al. also found no significant difference in their capacity to differentiate between de novo and advanced patients. These studies suggest that in clinical practice accuracy may be similar. On the other hand, comparative studies are limited, and thus, a conclusive statement with regard to the superiority of one scan over the other may not be possible.
Despite their relative (dis)advantages, in practice ‘couleur locale’, such as experience or availability, will most likely determine which of the two techniques is used.
27.3 Postsynaptic D2 Receptor Ligands
27.3.1 Indications
The primary and secondary indications for 123I-labelled iodobenzamide (123I-IBZM) are summarized in Table 27.3. As with FP-CIT, the basis of this summary will be briefly discussed.
Table 27.3
Parameters of the 123I-IBZM scan and 11C-raclopride PET scan
123I-IBZM SPECT | 11C-raclopride PET | |
|---|---|---|
Radionuclide | 123I, γ-rays, cyclotron produced | 11C, β-rays, cyclotron produced |
Drug-free interval | Yes | Yes |
Necessity of fasting | No | No |
Acquisition time | 50 min | 60 min |
Effective dose | Ca. 8 mSv | Ca. 4 mSv |
Dynamic images | No | Yes |
In 2002 a procedure guideline for using 123I-labelled dopamine D2 receptor ligands was published by the EANM task group. As ‘common indication’ was stated the differential diagnosis of parkinsonian syndromes: ‘a major indication is to distinguish PD from other parkinsonian syndromes (e.g. MSA or PSP)’ (Tatsch et al. 2002).
The EANM procedure guidelines for brain neurotransmission SPECT/PET using dopamine D2 receptor ligands, version 2 were published by Van Laere et al. in 2009 (Van Laere et al. 2009).
Aims of these guidelines were to assist nuclear medicine practitioners in making recommendations, performing, interpreting and reporting the results of clinical dopamine D2 receptor SPECT or PET studies, and to achieve a high quality standard of dopamine D2 receptor imaging, to increase the impact of this technique in neurological practice. The document was conceived as an update of the first guidelines for SPECT using D2 receptor ligands labelled with 123I and was guided by the views of the Society of Nuclear Medicine Brain Imaging Council and the individual experience of experts in European countries. ‘The main indication is the differentiation of Parkinson’s disease from other neurodegenerative parkinsonian syndromes characterized by loss of D2 receptors (e.g. multiple system atrophy and progressive supranuclear palsy). Several recent reports show that the use of D2 imaging alone may not be highly sensitive’ (Van Laere et al. 2009).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree