Introduction
More than 10,000 spinal cord injuries are diagnosed each year in the United States, with over half involving the cervical spinal cord. Multidetector computed tomography (CT) is recommended by the American College of Radiology as the first-line screening exam for any patient for whom spine injury cannot be excluded on clinical grounds alone. Coronal and sagittal spine reformats are also commonly incorporated into whole-body CT protocols for severe blunt trauma. CT is more cost-effective for initial cervical spine screening and appears to have a greater risk-benefit profile than plain radiography despite an order of magnitude greater radiation dose. This is largely due to the rate of missed injury on plain films, which can be higher than 50%. The use of magnetic resonance imaging (MRI) for spine clearance is controversial because CT is exquisitely sensitive for detection of unstable fracture patterns, and isolated signal abnormalities at MRI when bony relationships are normal are of questionable clinical significance. On the other hand, CT has little utility for directly evaluating the spinal cord and identifying extraaxial soft tissue pathology, owing to beam hardening from adjacent bony structures and poor contrast resolution. This chapter focuses primarily on issues pertaining to MRI of spinal cord injury (SCI).
MRI is frequently used after trauma to evaluate patients with persistent neck tenderness (not explainable by CT), myelopathy, absence of clinical improvement, and for suspected epidural hematomas or traumatic disk herniations that compress the spinal cord. MRI is also useful for prognosticating severity of SCI, determining surgical approach, confirming sufficient decompression of the spinal cord after surgery, evaluating progressive syrinx formation or other complications if recovery reverses in the chronic stage after injury, and guiding the duration of bracing in conservatively managed cases. Despite its widespread use, evidence supporting the use of MRI in the early acute setting for management purposes is not robust. The prognostic value of MRI for short- and long-term outcomes after SCI is relatively well established.
Spinal Cord Injury Mechanisms
Most fractures in both adult and pediatric patients with spinal cord injuries occur in the highly mobile cervical spine. These usually result from impulsive loading (i.e., whiplash). In adults the fulcrum of the cervical spine is at C5-C6, and unstable hyperflexion injuries tend to cluster around this motion segment ( Fig. 14-1 ). Hyperextension injuries have a higher center of rotation, often resulting from deceleration motor vehicle collisions with facial impact or in the elderly with face-planting after trivial falls.

In up to one third of patients, SCI occurs without skeletal or ligamentous disruption appreciable on CT. This has come to be called by the terms spinal cord injury without radiographic abnormality (SCIWORA) in pediatric patients and spinal cord injury without radiographic evidence of trauma (SCIWORET) in adults—in the latter to account for nontraumatic abnormalities usually related to spondylosis. In adults, SCI in this setting commonly manifests as central cord syndrome, in which the upper extremities are disproportionately affected ( Fig. 14-2 ). Young children have highly elastic spinal columns that can recoil back to normal alignment and exhibit no evidence of bone or discoligamentous injury despite severe SCIs, including transection. Some athletes involved in contact sports may be predisposed to “stinger syndrome” and “spinal cord concussion,” in which myelopathic symptoms are typically minor and resolve after short periods. Patients with congenitally narrowed spinal canals appear to be predisposed to SCI, but the positive predictive value of various measurement parameters of spinal stenosis appears to be limited.
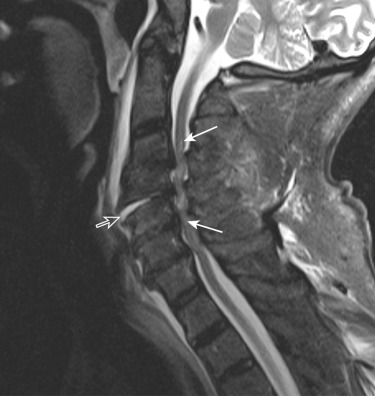
Extraneural findings that can benefit from decompression (e.g., traumatic disk herniations, epidural hematomas) are more common in older patients, especially in the setting of fused spine from ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis, chronic spondylotic changes, or prior spinal surgery ( Fig. 14-3 ). In those older than 60 years, profound residual deficits, morbidity, and high mortality are common after SCI.

MRI Protocol Considerations
1.5-tesla (T) and 3-T protocols vary considerably from institution to institution. Sagittal T2-weighted imaging or short tau inversion recovery (STIR) images are acquired with near ubiquity and are by far the most important from a prognostic standpoint because of their ability to visualize both the presence of spinal cord hemorrhage and the craniocaudal extent of edema. Sagittal proton density images can very clearly depict disruptions in the continuity of discoligamentous structures, but the added value of this sequence to T2-weighted imaging and STIR, which are also used for this purpose, remains unproven. Findings of ligamentous injury in the thoracolumbar and cervical spine at STIR have been shown to correlate well with findings at surgery, and STIR has the added benefit of identifying marrow edema and bone bruising due to fat suppression ( Fig. 14-4 ). Signal patterns useful for prognostication have not been established with axial images. Axial gradient echo (GRE) images are highly sensitive to susceptibility artifact from blood product and can depict areas of spinal cord hemorrhage with greater conspicuity than T2 (see Fig. 14-1B ). Nevertheless, the added prognostic value of GRE remains unknown, and GRE is incorporated in only about one fourth of protocols in published studies. Axial T2-weighted images have not been shown to have prognostic value but can identify clinically relevant lesions such as disk herniation and spinal cord compression that may alter management (see Fig. 14-3D ). Rapid MRI sequences such as fast imaging employing steady-state acquisition (FIESTA) have gradient echo properties but do not accentuate blood breakdown products, owing to the use of balanced steady-state pulse sequences. These may decrease motion artifact but add little to conventional T2 sequences. The use of advanced sequences such as diffusion-weighted imaging (DWI), diffusion tensor imaging (DTI), and susceptibility-weighted imaging (SWI) is not widespread, and data supporting their utility remains more controversial and less robust than in traumatic brain injury. There are diminishing returns from the acquisition of multiple sequences, and this must be weighed with considerations of safety, logistics, and resource allocation for patients who often require monitoring and anesthetic equipment and close oversight by healthcare personnel. Reducing the length of the total exam also increases the availability of the MR scanner.

Prognostic Significance of Mr Findings After SCI
SCI severity occurs along a spectrum that is encapsulated in a simplified four-pattern classification system introduced in the late 1980s and modified in the early 1990s. This has been shown in a handful of studies to correlate both with initial neurologic impairment (determined mostly using the American Spinal Cord Injury Association [ASIA] motor score) and degree of long-term improvement. Injuries are classified as (1) nonedematous cord, (2) edema spanning a single vertebral level, (3) edema spanning multiple levels, and (4) mixed hemorrhage and edema. After “normal” nonedematous spinal cord, which is seen in 10% to 20% of patients with SCI, single-level edema has the best prognosis. Approximately one fourth of patients with single-level edema recover normal motor and sensory function (ASIA E). Patients with diffuse edema ( Fig. 14-5 ) will almost invariably have permanent, at least partial, deficits with lower degrees of recovery. Those with diffuse edema who are initially ASIA A (complete motor and sensory deficit) will rarely show any meaningful improvement. Appreciable spinal cord hemorrhage is usually associated with complete motor and sensory loss and will rarely show meaningful improvement. Hemorrhage is rarely seen in the absence of edema. The term cord contusion has been variably used in the literature to refer to edema alone or to a specific pattern of edema with central isointensity or to denote frank spinal cord hemorrhage (i.e., synonymous with hemorrhagic contusion). Prognosis from spinal cord hemorrhage appears to be slightly better when less than 50% of the spinal cord cross-sectional area is involved. The length of hemorrhage does not correlate with injury severity, likely because of the uniformly poor outcome in these patients regardless of whether single or multiple levels are involved (see Fig. 14-1D ). For the same reason, complete cord transections are not included in prognostic grading scales ( Fig. 14-6 ). Cord expansion is seen with more severe injury resulting from accumulation of both intracellular and interstitial fluid above and below the injury. The utility of cord expansion as a predictor of outcome is controversial because the degree of enlargement can be limited by posttraumatic, degenerative, or congenital stenosis. Greater degrees of cord compression and canal compromise, best seen using axial T2 images, may correlate with worse prognosis. Spinal cord compression specifically by extraaxial hematoma may be another independent predictor of poor outcome, as is severity of prevertebral soft tissue injury, although this remains controversial.

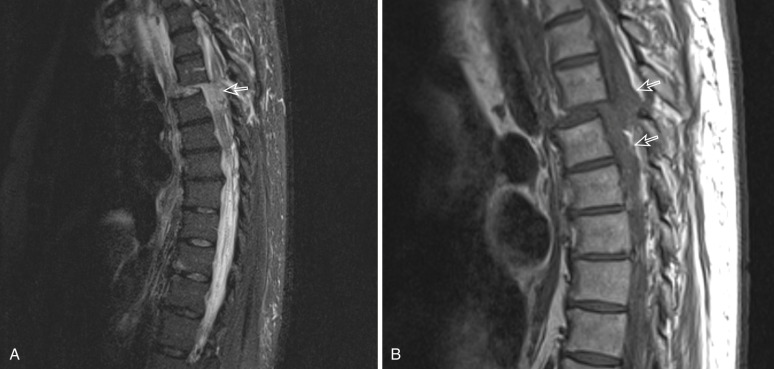
Evolution of Spinal Cord Injury—Findings at MRI
Because of the small caliber of the cord and narrow space within the spinal canal, even small foci of hemorrhage within the spinal cord are associated with devastating neurologic outcomes and poor improvement over time. As such, SCI is not generally thought of in the discrete terms of primary and secondary injury that are used to characterize traumatic brain injury. The relationship between evolution of signal abnormality and time after injury has been studied, but major discrepancies between exam and electrophysiologic testing and MRI appearance are common. The ideal time interval for performing MRI after initial injury remains controversial. Surgical decompression within 24 hours for SCI patients and “very early” decompression within 12 hours for those with incomplete cervical SCIs has been recommended based on a worldwide survey of 971 spine surgeons. Although these recommendations would seem to support MRI in the early acute phase, it is not uniformly practiced at many centers. Bondurant et al. previously recommended that MRI be performed within 24 to 72 hours after trauma.
Generally MRI plays an ancillary role to neurophysiologic testing, with additional prognostic information provided by injury morphology, as described in the previous section. As SCI transitions from acute to chronic phases, it is important to understand expected secondary morphologic changes resulting from injury evolution in the posttraumatic lesion area.
Initially, edema with or without hemorrhage spreads cranially and caudally from the injury epicenter ( Fig. 14-7 ). Shortly after injury, spinal cord hemorrhage is usually appreciated as low signal on T2 and edema as high signal. Extraneural blood may take only hours to develop high signal from methemoglobin (see Fig. 14-4B ), whereas intraneurally this may take days to weeks, limiting the utility of T1-weighted imaging in the early acute phase after injury ( Fig. 14-8 ). Sagittal T2-weighted and STIR images offer a global view of both the cord and discoligamentous structures along the full extent of the imaged spine. Acute injury is followed by a second subacute stage marked by regression of edema and hemorrhage, during which the transverse diameter of the cord may subside to normal or shrink. In the final chronic stage, a posttraumatic cyst may form. Posttraumatic cysts are synonymous with syringomyelia or syrinx. These usually follow the signal intensity of cerebrospinal fluid (CSF) and are well defined ( Fig. 14-9 ). They result from rents in the ependymal lining of the central canal but are not covered by ependyma. Syringomyelia can sometimes be difficult to differentiate from hydromyelia (dilation of the central canal), and in such cases the term syringohydromyelia is used. Once a posttraumatic cyst has fully formed—usually within 1 year after injury—the cyst and spinal cord area are usually stable. In some instances posttraumatic cyst enlargement is observed ( Fig. 14-10 ), but this does not necessarily correlate with symptomatology. Posttraumatic syrinx develops in 5% to 8% of SCI. Tethering and syrinx progression are suspected if the myotomes affected become more rostral. Surgical intervention may be warranted purely on the basis of MR findings if the rostral extent threatens to encroach on the brainstem.


Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree