Fig. 46.1
Comparison of treatment plans for 1-, 3-, and 5-fraction SBRT
The long-term patient-reported outcomes data collected prospectively for hypofractionated SBRT at MDACC was reported by Wang et al. [3] on 149 patients with mechanically stable spinal metastases. Fractionated SBRT was found to be effective therapy for tumor control and symptomatic improvement. Patients received a total dose of 27–30 Gy, delivered in 3 fractions. With a median follow-up of 15.9 months, the progression-free survival after SBRT was 80.5 % at 1 year and 72.4 % at 2 years. The number of patients reporting no pain from bone metastases increased from 26 to 54 % 6 months after SBRT. In addition, there was a statistically significant reduction in multiple symptoms including disturbed sleep, drowsiness, sadness, fatigue, distress, lack of appetite, nausea, and difficulty remembering.
On the other hand, single-fraction SBRT has the advantage of convenience to the patient and treatment team and is thus attractive, if and only if it can be delivered safely. The spinal cord tolerance may be better defined for single-fraction SBRT based on extrapolation of data from radiosurgery to the optic chiasm of approximately 8 Gy making it easier to achieve complete target volume coverage. This can be appreciated by examining dose-volume histograms from treatment plans representing 30 Gy in 5 fractions, 27 Gy in 3 fractions, and 18 Gy in 1 fraction (Fig. 46.2). Furthermore, single-fraction SBRT follows the successful paradigm established in the arena of intracranial radiosurgery and may lead to superior local control. Single-fraction SBRT may be preferable for single tumors that are small in volume and anatomically favorable in terms of greater distance from the spinal cord. Fractionated SBRT may be preferable for spinal tumors with paraspinal components spanning multiple vertebral body levels or with large prevertebral components juxtaposed to small bowel, because the biological dose to surrounding normal tissues can be minimized to a greater degree. For tumors surrounding the spinal cord, involving the epidural space, or causing compression of the thecal sac, it may be prudent to request that the neurosurgeon resects the disease in close proximity to the spinal cord so that a more favorable anatomic configuration can be obtained for SBRT. A comparison of modalities including single-fraction and hypofractionated SBRT, conventional radiotherapy, and surgery is shown in Fig. 46.1.
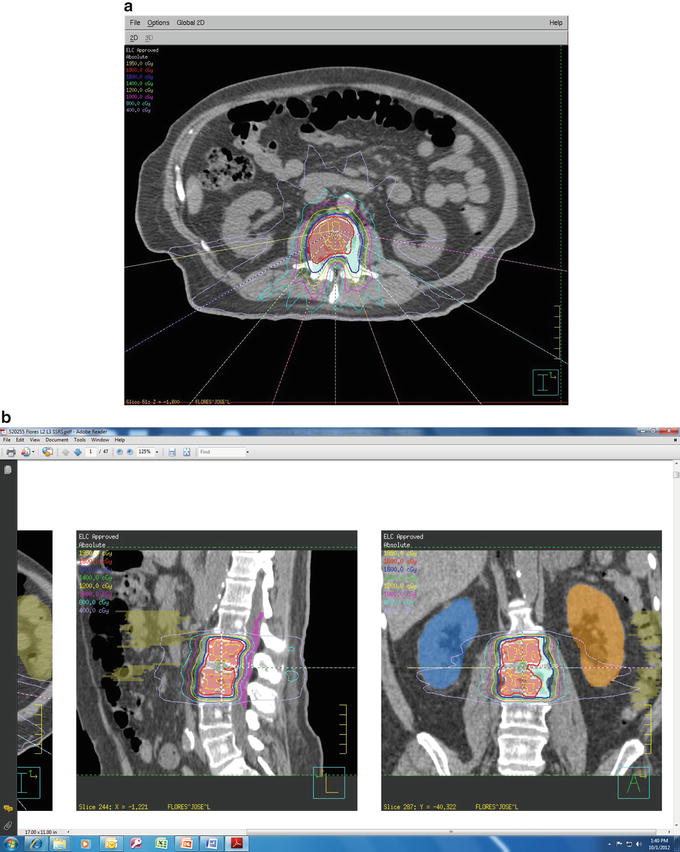

Fig. 46.2
Single-fraction dose delivering 18 Gy to nonrenal cell spinal metastases (a) axial and (b) sagittal
Table 46.1
Comparison of various spinal metastasis treatments
Single-fraction SBRT | Hypofractionated SBRT | Conventional radiotherapy | Surgery | |
|---|---|---|---|---|
Safety | Unforgiving. No possibility for correction after delivery of treatment; spinal cord tolerance extrapolated from optic chiasm; may be less appropriate in previously irradiated patients | Some compensation possible if initial setup error made; spinal cord tolerance for hypofractionated radiotherapy ill-defined; good approach for setting up new programs during learning curve; may be safer for previously irradiated patients | 30 Gy in 10 fractions has track record of safety in treating a large number of patients worldwide | Usual potential risks of surgery including wound infection, bleeding, damage to nerves, and spinal cord |
Efficacy | Theoretically may have greater ability than hypofractionated SBRT to overcome radioresistance especially for renal cell carcinoma, melanoma, and sarcoma by getting over shoulder of survival curve | Needs to be prospectively compared with single-fraction SBRT and conventional radiotherapy | Mainstay for palliation of spinal metastases; limited by spinal cord tolerance so dose may be inadequate; reirradiation usually not possible | Rapid resolution of neurologic symptoms due to spinal cord compression or pain caused by mechanical instability |
Convenience | Ultimate in convenience to patient and treatment team, must be balanced against potential risks | Greater expenditure of resources required for labor-intensive procedure; may be required during learning curve or for previously irradiated patients near cord tolerance | Can start immediately, easy to give, widely available | Postoperative recovery time, wound healing, rehabilitation time to achieve ambulation |
Cost-effectiveness | May be less costly than fractionated SBRT from a resource standpoint, and therefore more cost-effective if efficacy is proved to be equivalent | More costly due to multiple iterations of the same procedure; however, this cost may need to be incurred initially by new programs to ensure patient safety until track record for safety established | Cost-effective and appropriate for majority of diffuse spinal metastases involving multiple levels | Costly but definite indications for spinal cord compression and salvaging previously irradiated metastases |
Published Clinical Results
At selected centers, SBRT programs are becoming more mature, and there is recognition of the importance of credentialing system that is being put into place at many centers. Therefore, the opportunity to collect larger experiences or long-term clinical data is now happening. Bilsky et al. from Memorial Sloan Kettering reported on their initial clinical experience in treating 16 paraspinal tumors with intensity-modulated stereotactic radiotherapy. Metastatic tumors received a dose of 20 Gy in 4–5 fractions while limiting the spinal cord to a maximum dose of 6 Gy. Of the 15 patients who underwent radiographic follow-up, 13 demonstrated either no interval growth or a reduction in tumor size in a median follow-up period of 12 months (range, 2–23 months) [4]. Our group at The University of Texas MD Anderson Cancer Center (MDACC) reported on 15 consecutive patients with metastatic spinal disease who underwent 75 treatments involving 90 isocenter setups on a phase I clinical trial involving intensity-modulated, computed tomography (CT) image-guided SBRT. Patients uniformly received 30 Gy in 5 fractions while the spinal cord was constrained to a maximum dose of 10 Gy [5]. The procedure was technically feasible to perform in all patients, and no neurologic toxicity was observed in any patient with a median follow-up time of 9 months (range, 6–16 months). Because SBRT for spinal metastases is usually given in the context of palliation and avoiding spinal cord damage is paramount, we initially chose to prescribe a hypofractionated dose of 30 Gy in 5 fractions on alternating days to allow for sufficient sublethal damage repair while conservatively limiting the spinal cord dose to 10 Gy. It is important to note that treatment planning programs approximate doses to critical structures and are limited by the accuracy of beam data taken by ion chamber measurements, which is affected by the size or volume of the ion chamber, and whether leakage between the leaves used in multileaf collimators and leaf shape are taken into account. Recognizing that treatment plans may over- or underestimate the actual doses delivered, it is prudent to conservatively limit doses to the spinal cord. A dose of 10 Gy in 5 fractions is considered clinically insignificant. The biologic equivalence (BED2Gy) of 30 Gy in 5 fractions is 40 Gy for early responding tissues assuming an α/β of 10 Gy and 54–64 Gy assuming an α/β of 1.5–3 Gy for the spinal cord. With the increased confidence of our safety and setup data demonstrating consistent daily patient setups within 1 mm of isocenter [5], we have proceeded to shorten our treatments to a total dose of 27 Gy in 3 fractions on alternating days to allow for sufficient repair while limiting the spinal cord dose to 10 Gy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








