Fig. 1
Normal gallbladder histology. The mucosa consists of a single layer of tall columnar epithelial cells and underlying lamina propria. Underneath the mucosa are a smooth muscle layer and a perimuscular connective tissue layer. The serosa is not shown in this figure
1.
The mucosa, which consists of a single layer of tall columnar epithelial cells basally anchored to a basement membrane, and underlying lamina propria. In the region of the neck, mucin-secreting glands surround the epithelium.
2.
A smooth muscle layer, most prominent in the region of the neck.
3.
Perimuscular connective tissue.
4.
Serosa.
It is important to note that the gallbladder has no true muscularis propria or muscularis mucosa. It is also important to note that serosa is not present along the hepatic side. Instead, the perimuscular connective tissue is rather continuous with the interlobular hepatic connective tissue in this region; this facilitates tumor extension into the liver [66].
Gallbladder carcinoma has known association with gallstones, which are found in 80 % of carcinomas and are considered a risk factor for gallbladder carcinoma [24]. Nevertheless, the overall incidence of gallbladder carcinoma in patients with cholelithiasis is still low (<0.2 %) [65]. There is also an association between gallbladder carcinoma and an abnormal choledochopancreatic junction. Gallbladder carcinoma in this setting develops in younger (10 years younger) patients in the absence of gallstones [56, 108]. Therefore, when gallbladder carcinoma occurs in the absence of gallstones, the pathologist should recommend evaluation of the choledochopancreatic junction. Primary sclerosing cholangitis (PSC) is another risk factor for gallbladder carcinoma. In PSC, gallbladder adenocarcinoma often arises in a background of intestinal metaplasia and dysplasia. In fact, data support a metaplasia–dysplasia–carcinoma sequence in gallbladder carcinogenesis in PSC patients [62]. There is conflicting data on the association between calcified (porcelain) gallbladder and gallbladder carcinoma [2, 82, 100]. The location of calcifications may be an important determinant of carcinoma risk, whereas no carcinoma was observed in gallbladders with diffuse intramural calcification, and cancer incidence was significantly increased in gallbladders with selective mucosal calcification [93]. In any case, it is advisable to carefully evaluate calcified gallbladder specimens and adequately sample them to exclude the possibility of carcinoma. Other risk factors for gallbladder carcinoma include ulcerative colitis, familial adenomatous polyposis (FAP), and certain chronic infections [2].
2.2 Gross and Microscopic Features
Gallbladder carcinomas can involve the fundus (60 %), body (30 %), and neck (10 %) [2]. Although early stage tumors can be difficult to recognize grossly, most gallbladder carcinomas produce a grossly recognizable mass. They can appear as localized or diffuse wall thickening, a raised plaque, a submucosal nodule, a polypoid growth, or a combination of all these patterns (Fig. 2). Infiltrative growth is often present and is considered an independent predictor of recurrence in gallbladder carcinoma [81]. Certain tumor subtypes have a characteristic gross appearance. For instance, papillary carcinomas have a sessile polypoid or cauliflower-like gross appearance, and mucinous tumors have a mucoid/gelatinous cut surface.
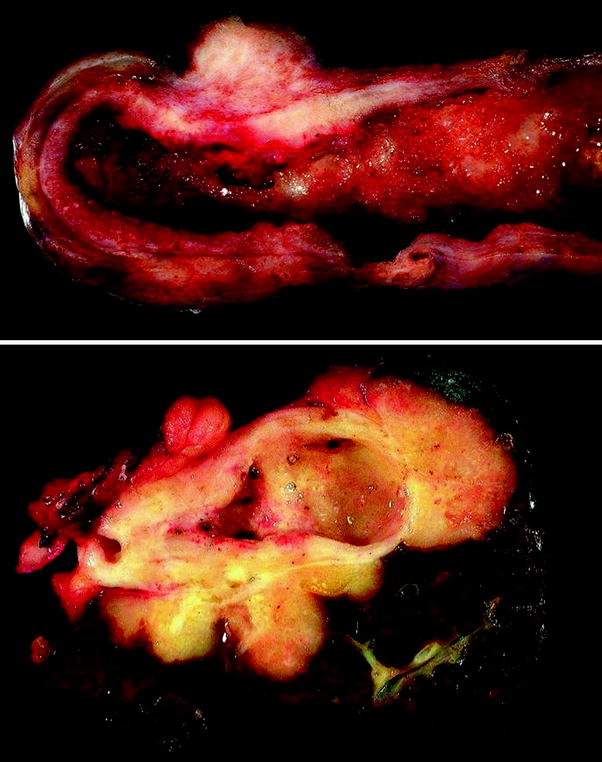

Fig. 2
Gross features of gallbladder carcinoma. Upper carcinoma involving the gallbladder fundus and body and forming a transmural mass with diffuse wall infiltration. Lower gallbladder carcinoma infiltrating surrounding liver
Microscopically, carcinoma in situ (CIS) is defined as carcinoma that is confined to the epithelium, usually growing upward or laterally, without evidence of stromal invasion. Histologic subtypes of CIS include intestinal type, signet ring cell type, and squamous cell CIS. When CIS is found in the gallbladder, multiple sections should be taken to exclude invasion. If no invasion is found and the margins are free, the survival is excellent (virtually 100 % at 5 years) and no further treatment is necessary [2].
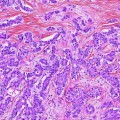
More than 98 % of malignant gallbladder tumors are carcinomas [2], and adenocarcinoma is the most common type (87 %) [28] of invasive carcinoma. Adenocarcinoma is characterized by infiltrative growth of variably sized and irregularly shaped glands, usually with desmoplastic response (Fig. 3). It is important to differentiate adenocarcinoma from other benign entities such as adenomyomatous hyperplasia [3]. Variants of adenocarcinoma include the following:
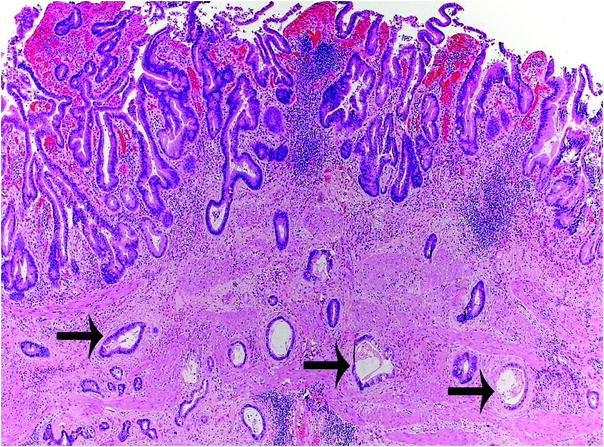

Fig. 3
Histologic features of gallbladder carcinoma. Invasive adenocarcinoma is characterized by infiltrative growth of variably sized and irregularly shaped glands (arrows), with desmoplastic response
1.
Papillary adenocarcinoma is considered a variant of well-differentiated adenocarcinoma, showing a predominant polypoid/intraluminal rather than invasive growth, which results in a relatively improved prognosis (best survival rates of gallbladder carcinoma). Papillary adenocarcinoma can be invasive or noninvasive. Because noninvasive papillary carcinoma has such an excellent prognosis [40, 41, 75], extensive sampling to exclude invasion is recommended.
2.
3.
4.
5.
Signet ring cell adenocarcinoma constitutes approximately 3 % of gallbladder carcinomas.
Other carcinomas known to involve the gallbladder include adenosquamous carcinoma: (5–9 % of gallbladder carcinoma) [2, 28, 76], which consists of a mixture of squamous cell carcinoma and adenocarcinoma, squamous cell carcinoma (1–7 % of gallbladder carcinoma) [28], small cell carcinoma (4 % of gallbladder carcinoma), and undifferentiated carcinoma. Small cell carcinoma is morphologically similar to pulmonary small cell carcinoma and has a highly aggressive clinical behavior [2, 4, 84]. This histologic type should be reported even if it is a minor component of a gallbladder carcinoma, as such combined tumors still behave aggressively. Undifferentiated carcinoma has four variants, the commonest of which are spindle/giant cell type [2, 16] and small cell type [2]. In undifferentiated carcinoma, good sampling can unveil a better-differentiated invasive carcinoma component or CIS in most cases. Recording the histologic type is important, since some types (e.g., papillary carcinoma) have a better survival rate, whereas others (e.g., undifferentiated carcinoma and small cell carcinoma) have the worst prognosis (patients usually survive less than 1 year) [2]. This is also important for treatment purposes; for instance, chemotherapeutic choice for small cell carcinoma is different from that of adenocarcinoma. When there is more than one histologic pattern, it is recommended to record all patterns in the pathologic diagnosis. It is also recommended to record the tumor’s histologic grade because it is a significant predictor of patient outcome [11, 28] and an independent predictor of recurrence in gallbladder carcinoma [81]. Grading adenocarcinoma can be evaluated by assessing the glandular versus solid component of the tumor histologically (grade 1: >95 % glands, grade 2: 50–95 % glands, grade 3: <49 % or less glands, and grade 4: undifferentiated). Gallbladder adenocarcinoma is most commonly in grades 1 and 2 [28].
2.3 Staging
TNM stage is a significant predictor of patient outcome [11, 20, 28, 64, 105]. In fact, disease stage is the single most important factor in determining patient survival at the time of diagnosis. The depth/extent of tumor invasion has prognostic value; the incidence of lymph node and distant metastasis increases progressively with increased T stage [66, 88]. Invasion beyond one-third of the subserosal layer carries significantly increased risk of tumor spread and may warrant para-aortic lymph node sampling [88]. Tables 1 and 2 outline the current TNM and overall staging of gallbladder carcinoma. Of note, cystic duct carcinomas are now included in the gallbladder TNM classification scheme. Carcinomas of the gallbladder are staged according to their depth of invasion into the wall and extension into adjacent structures. Tumors confined to the gallbladder are classified as T1 or T2 depending on the depth of tumor invasion. Tumors extending beyond the gallbladder wall, through the serosa, and/or involving adjacent structures are designated T3. Because invasion of hilar structures usually renders the cancer unresectable, such tumors are designated T4.
Primary tumor (T) | |
TX | Cannot be assessed |
TO | No evidence of primary tumor |
Tis | Carcinoma in situ |
T1 | Tumor invades lamina propria or muscular layer |
T1a | Tumor invades lamina propria |
T1b | Tumor invades muscle layer |
T2 | Tumor invades perimuscular connective tissue; no extension beyond serosa or into liver |
T3 | Tumor perforates serosa (visceral peritoneum) and/or directly invades the liver and/or one other adjacent organ or structure, such as the stomach, duodenum, colon, pancreas, omentum, or extrahepatic bile ducts |
T4 | Tumor invades main portal vein or hepatic artery or invades 2 or more extrahepatic organs or structures |
Regional lymph nodes (N) | |
NX | Cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Metastases to nodes along the cystic duct, common bile duct, hepatic artery, and/or portal vein |
N2 | Metastases to periaortic, periaortic, superior mesentery artery, and/or celiac artery lymph nodes |
Distant metastasis (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis |
Overall pathologic stage | |||
|---|---|---|---|
Stage 0 | Tis | N0 | M0 |
Stage 1 | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage IIIA | T3 | N0 | M0 |
Stage IIIB | T1–3 | N1 | M0 |
Stage IVA | T4 | N0–1 | M0 |
Stage IVB | Any T | N2 | M0 |
Any T | Any N | M1 | |
Because the gallbladder has a thin wall, tumors extend quickly into the perimuscular connective tissue, gaining access to a rich vascular and lymphatic network, and facilitating tumor spread. Hence, gallbladder carcinoma often metastasizes early, even before the diagnosis is made. Gallbladder tumors can involve the liver, stomach, duodenum, or colon by direct extension, can implant on peritoneal surfaces, or metastasize to regional lymph nodes and distant organs. Regional lymph node metastasis is a significant independent predictor of patient survival [25, 86, 107]. It occurs frequently early in gallbladder cancer (found in 19–50 % of patients at the time of diagnosis) [25]. Regional lymph nodes are limited to the hilar (cystic, pericholedochal, hepatic artery, and portal vein) lymph nodes staged as N1 and other regional (para-aortic, pericaval, superior mesenteric artery, and celiac artery) staged as N2 [51]. Hence, involvement of peripancreatic nodes along the body and tail of the pancreas is considered distant metastases (M1). In addition to topographic location, which determines the N stage, the number of involved lymph nodes is also important in predicting survival [31]. Therefore, the surgical pathologist should document the number of examined and number of positive lymph nodes. Accurate N staging requires sampling of a minimum of three regional lymph nodes for microscopic examination [66]. In addition to histologically evident metastases, one study showed that immunohistochemically detected micrometastases, regardless of size, are associated with poor survival and should be designated N1 [86]. Because these findings are limited to one study and remain unvalidated by larger series, the data are currently insufficient to recommend routine immunohistochemical evaluation of regional lymph nodes. Hence, routine assessment of regional lymph nodes is currently limited to H&E sections. Distant metastases are found in 57 % of patients with gallbladder carcinoma [28]. They most often involve the liver, peritoneum, and lung [66]. The liver is involved in 13–70 % of patients at the time of surgery, either by direct extension (most common) or by metastasis [25].
2.4 Other Important Pathologic Features
Additional important pathologic features in gallbladder carcinoma include perineural invasion, angiolymphatic invasion, and surgical margin status. Perineural invasion occurs in most (71 %) gallbladder carcinomas and is associated with significantly lower 5 year survival rate [20], but there is conflicting data in regard to its value as an independent predictor of survival [7, 20, 86, 105]. Perineural invasion is also significantly associated with extrahepatic bile duct invasion [107]. Likewise, angiolymphatic invasion is a significant prognostic factor in gallbladder carcinoma and its absence is significantly associated with long-term survival [7, 20, 105]. In fact, vascular invasion seen histologically has the effect of reducing outcome to the next disease stage. It is therefore important to report it. The surgical margin status is a significant prognostic factor in gallbladder carcinoma [105]; long-term survival is significantly associated with complete resection [11].
Gallbladder carcinoma is often identified in laparoscopically resected gallbladders. Laparoscopic cholecystectomy carries a risk of cancer implantation/dissemination; it should not be performed in gallbladders with high suspicion of malignancy [9, 34, 68, 92]. Nevertheless, invasive carcinoma, undetected clinically, is occasionally discovered first by the pathologist evaluating laparoscopic cholecystectomy specimens. These tumors are often low stage (T1 or T2) [23], but specimen disruption can complicate pathologic staging. Port-site recurrence is also a serious complication of gallbladder cancers resected laparoscopically [80] usually treated by follow-up surgery, which includes port-sites excision if the cancer is resectable (T1 and T2) [35]. Because carcinoma can be found incidentally, careful gross examination and mandatory histologic evaluation (especially if the gallbladder wall is thickened) are essential in detecting such tumors. In addition, when a polyp or a distinct lesion is present, the entire lesion should be examined microscopically.
3 Extrahepatic Bile Ducts
3.1 Normal Anatomy and Predisposing Conditions
Bile flows from the liver through the hepatic ducts, which join to form the common hepatic duct (0.8–5.2 cm in length), which in turn descends along the lateral aspect of the hepatoduodenal ligament, and joins the cystic duct (0.4–6.5 cm in length) to form the common bile duct (1.5–9 cm in length). The common bile duct passes posterior to the duodenum, traverses the head of the pancreas, and finally opens into the second part of the duodenum through the major papilla (papilla of Vater). The common bile duct is divided into four parts: supraduodenal (longest and most accessible), retroduodenal, pancreatic, and intraduodenal. The walls of the extrahepatic bile ducts are very thin (<1.5 mm in thickness), predisposing to rapid invasion of malignant tumors into the periductal tissue, gaining access to a rich vascular and lymphatic network, which facilitates tumor spread. The microscopic anatomy of all extrahepatic bile ducts is essentially the same (Fig. 4). They consist of:
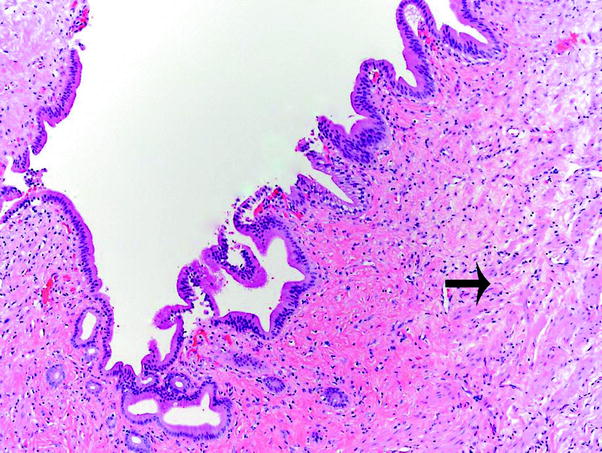

Fig. 4
Normal extrahepatic bile duct histology. The mucosa consists of columnar epithelium with small longitudinal folds and a subepithelial layer containing elastic and collagen fibers. Scattered small bundles of smooth muscle (arrow) underlie the mucosa
1.
Mucosa, which consists of tall columnar epithelium with small longitudinal folds and a subepithelial layer containing elastic and collagen fibers.
2.
Scattered small bundles of smooth muscle in the ductal wall parallel with the lumen. This layer becomes prominent in the common bile duct near the sphincter of Oddi.
3.
Periductal layer of loose connective tissue.
Intramural mucous glands are present and increase toward the distal part; they open into the lumen in small pits (the sacculi of Beale).
Extrahepatic bile duct carcinoma can be associated with ulcerative colitis, PSC (carcinoma usually occurs at younger age), abnormal choledochopancreatic junction, choledochal cysts (carcinoma usually occurs at younger age), and certain longstanding biliary infections [2, 19, 32, 47, 52, 58, 60, 67, 69, 71, 97]. The dysplasia–carcinoma sequence appears to be the usual pathway for development of invasive carcinoma of the extrahepatic bile ducts, especially in ulcerative colitis and PSC [2].
3.2 Gross and Microscopic Features
Approximately 54 % of extrahepatic bile duct carcinomas are perihilar and 42 % are distal (see Sect. 2.3) [26]. Distal bile duct carcinomas should be distinguished from pancreatic carcinomas because they have a better outcome [33, 53]. Grossly, extrahepatic bile duct carcinomas can be constrictive (sclerosing), nodular, polypoid/papillary, diffusely infiltrative, or mixed sclerosing/nodular [99, 102] (Fig. 5). Sclerosing tumors are the most common. They usually have a perihilar location and show diffuse infiltration and fibrosis of periductal tissue, producing a very firm and thickened duct. Papillary tumors are common in the distal extrahepatic bile ducts and are soft and friable; they produce little if any mural invasion and therefore have a favorable outcome [48]. Similar to the gallbladder, certain tumor subtypes have a characteristic gross appearance. For instance, papillary carcinomas have a polypoid appearance and mucinous tumors have a gelatinous cut surface.
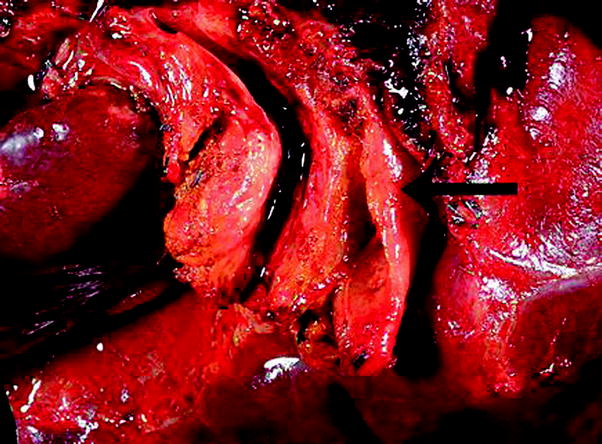

Fig. 5
Gross features of perihilar bile duct carcinoma. Perihilar carcinoma characterized by diffuse thickening of the extrahepatic bile ducts
Microscopically, the morphologic criteria for classification and grading of in situ and invasive carcinoma of the extrahepatic bile ducts are similar to those in the gallbladder. Like in the gallbladder, the vast majority (>90 %) of extrahepatic bile duct carcinomas are adenocarcinoma [48] (Fig. 6). Papillary and grade 1 adenocarcinomas are associated with polypoid and nodular macroscopic types and are often located in the upper portion of the extrahepatic biliary tree, whereas grade 2–3 adenocarcinomas are associated with constrictive (sclerosing) macroscopic type and tend to involve the lower biliary tree. The latter carcinomas have higher rates of angiolymphatic invasion and lymph node metastasis [106]. Papillary carcinomas represent 3–23 % of extrahepatic bile duct tumors [27, 49]. They are larger, more well-differentiated and have an earlier stage and significantly improved survival (especially if noninvasive) compared to nodular sclerosing type adenocarcinoma [49]. In fact, noninvasive and minimally invasive papillary carcinomas have the best prognosis among extrahepatic bile duct carcinoma. In contrast, squamous cell carcinoma, undifferentiated carcinoma, signet ring cell carcinoma, and small cell carcinoma have the worst prognosis [39]. Mucinous, adenosquamous, and squamous cell carcinomas represent 4, 2, and 2 %, of extrahepatic carcinomas, respectively [27]. The frequency of small cell carcinoma and undifferentiated carcinoma is lower in the extrahepatic bile ducts than in the gallbladder. Similar to gallbladder carcinoma, histologic grade is a useful prognostic marker in extrahepatic bile duct carcinoma [26, 29, 39, 50].
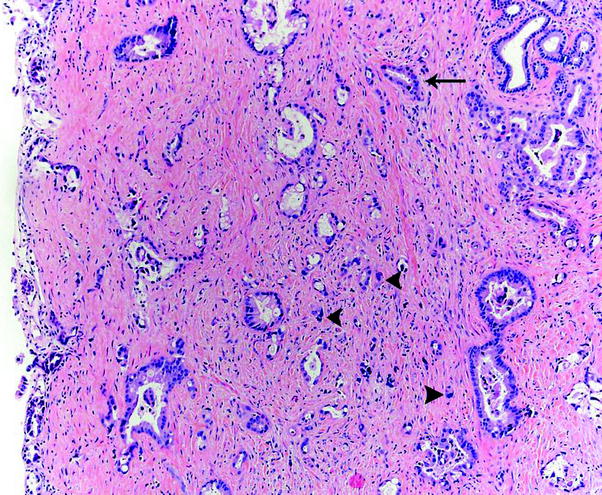

Fig. 6
Histologic features of bile duct carcinoma. Invasive adenocarcinoma is characterized by infiltrative growth of variably sized and irregularly shaped glands (arrow) as well as single cells (arrowheads), with desmoplastic response
3.3 Staging
Disease stage is an important prognostic indicator [37, 39, 45, 70]. For instance, increased depth of invasion (T stage) carries poor outcome [43]; the frequency of lymph node metastasis increases with increased tumor depth [42, 79]. In addition, gross (not microscopic) portal vein invasion by perihilar carcinoma is independently associated with poor prognosis [29]. Hence, invasion of the portal vein and regional lymph node metastasis should be weighted equally [77] (both stages III–IV in perihilar bile duct carcinomas). Extrahepatic bile duct carcinomas invade through the thin duct wall early and hence spread early by direct extension into adjacent structures, including the portal vein, hepatic artery, perihilar liver parenchyma, and pancreas. Distal tumors can also involve the colon, duodenum, and rarely the stomach.
It is important to note that different T and N criteria now exist for staging perihilar and distal extrahepatic bile ducts. Tables 3, 4, 5, and 6 outline the current TNM and overall staging of perihilar and distal extrahepatic bile ducts, respectively. Perihilar tumors are defined as those involving the hepatic duct bifurcation or extrahepatic ducts proximal to the origin of the cystic duct, whereas distal tumors are defined as those involving the biliary system between the cystic duct–common bile duct junction and the ampulla of Vater. The distal bile duct staging system also applies to choledochal cysts. In contrast to gallbladder cancer stating, only T1 lesions are confined to the bile duct, whereas T2 lesions invade beyond the wall of the bile duct and can involve the liver in the perihilar location. To differentiate between T1 and T2 tumors, definition of the boundaries of the bile duct wall is important. T1 lesions are best defined as tumors within the outermost part of the muscle layer or fibrous tissue, whereas T2 tumors involve the area of large clusters of adipose tissue and beyond [45]. Despite their relatively small size, most bile duct tumors have usually spread to adjacent organs and many have spread to regional lymph nodes by the time of diagnosis. Therefore, early (T1) extrahepatic bile duct carcinomas are uncommon (10 % of resected specimens). They are frequently asymptomatic and most show intraductal-growth, with approximately one-third representing papillary carcinoma [17]. In perihilar tumors, unilateral vascular (portal vein or hepatic artery) involvement is now designated T3, whereas bilateral involvement of vascular structures, bilateral tumor expansion into secondary biliary radicals, or extension into secondary biliary radicals with contralateral vascular invasion is designated T4. Invasion of adjacent structures besides the celiac axis or superior mesenteric artery is classified as T3 tumors in the distal extrahepatic bile ducts. In these T3 lesions, deep pancreatic invasion (more than 1 mm) is a significant and independent determinant of patient prognosis [44].
Primary tumor (T) | |
TX | Cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ |
T1 | Tumor confined to the bile duct, with extension up to the muscle layer or fibrous tissue |
T2a | Tumor invades beyond the wall of the bile duct to surroundings adipose tissue |
T2b | Tumor invades adjacent hepatic parenchyma |
T3 | Tumor invades unilateral branches of the portal vein or hepatic artery |
T4 | Tumor invades main portal vein or its branches bilaterally; or the common hepatic artery; or the second-order biliary radicals bilaterally; or unilateral second-order biliary radicals with contralateral portal vein or hepatic artery involvement |
Regional lymph nodes (N) | |
NX | Cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Regional lymph node metastasis (including nodes along the cystic duct, common bile duct, hepatic artery, and portal vein) |
N2 | Metastasis to periaortic, pericaval, superior mesentery artery, and/or celiac artery lymph nodes |
Distant metastasis (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis |
Overall pathologic stage | |||
|---|---|---|---|
Stage 0 | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage II | T2a-b | N0 | M0 |
Stage IIIA | T3 | N0 | M0 |
Stage IIIB | T1-3 | N1 | M0 |
Stage IVA | T4 | N0–1 | M0 |
Stage IVB | Any T | N2 | M0 |
Any T | Any N | M1 | |