Magnetic resonance (MR) imaging is one of the most important paraclinical tools for the diagnosis of multiple sclerosis (MS), and monitoring of disease progression and treatment response. This article provides clinicians and neuroradiologists caring for children with demyelinating disorders with a suggested standard MR imaging acquisition and reporting protocol, and defines a standard lexicon for lesion features typical of MS in children. As there is considerable overlap between the MR imaging features of pediatric- and adult-onset MS, the recommendations provided herein may be of relevance to radiologists and clinicians caring for adults with multiple sclerosis.
Key points
- •
The value of MR imaging in the diagnosis and clinical management of children with multiple sclerosis (MS) rests in part on the use of a consistent and standard imaging protocol.
- •
Adopting a standard lexicon and reporting scheme for pediatric central nervous system (CNS) demyelination will be valuable for implementing structured reporting into radiological practice.
- •
We propose a standard MR imaging acquisition and reporting protocol based on the pediatric MS literature and experience and insights gained from following children with acute CNS demyelination and MS in the Canadian Pediatric Demyelinating Disease Network.
- •
Our goal is to provide a framework within which to refine the protocol to enhance its national and international applicability.
Introduction
Magnetic resonance (MR) imaging is perhaps one of the most important paraclinical tools for the diagnosis of multiple sclerosis (MS) and for monitoring disease progression and treatment response. However, the value of MR imaging in informing clinical management of MS patients largely depends on the use of a consistent and standard imaging protocol. The goals of MR imaging in MS include: confirmation of an MS diagnosis before a second clinical attack in individuals with an acute demyelinating syndrome according to dissemination in time and space criteria ; exclusion of alternative diagnoses ; and prediction of outcome. In addition, clinicians caring for patients with MS rely on comparison of serial scans to qualitatively evaluate the rate of new lesion accrual for diagnosis, to inform on treatment decisions, and to monitor disease evolution apparent by formation of confluent lesions and atrophy. A standard MR imaging acquisition protocol and reporting method will improve the accuracy and reliability of MR imaging evaluation among radiologists and clinicians within and across centers, and will aid the clinician in managing patients with MS.
An expert panel of adult MS radiologists and neurologists within the Consortium of MS Centers published a consensus-based MR imaging protocol for use in the investigation and monitoring of patients with MS. However, the panel indicated that the applicability of the protocol to pediatric-onset MS requires further study. Although many of the MR imaging features of MS in children may overlap with those of adults, specific considerations unique to the imaging of children and the distinct MR imaging features of MS in prepubertal children may necessitate a revised MR imaging protocol for pediatric MS.
The goal of this article is to provide clinicians and neuroradiologists caring for children with demyelinating disorders with a suggested standard MR imaging acquisition and reporting protocol, and to define a standard lexicon for lesion features typical of MS in children. Only conventional MR imaging sequences used in routine clinical practice and standard to most scanners are discussed; advanced MR imaging techniques are being increasingly applied in MS research, but are not yet applicable or feasible in the clinical setting. As considerable overlap exists between the features of pediatric-onset and adult-onset MS, these recommendations may be of relevance to adult clinicians and radiologists. The recommendations are based on recent MR imaging studies conducted in pediatric MS and on the expertise of pediatric MS neurologists and neuroradiologists at The Hospital for Sick Children (Toronto, Canada), the lead center for the Canadian Pediatric Demyelinating Disease Network, a prospective cohort study in which standardized clinical and MR imaging data and biological samples are collected at onset and serially in children with incident demyelination.
Unique considerations in MR imaging of children
There are several considerations to be mindful of when imaging the child being investigated for MS.
- 1.
In very young children, primary myelination is not yet complete. Use of T2-weighted sequences and, to a greater extent, fluid-attenuated inversion recovery (FLAIR) imaging, is not optimal for the assessment of the presence of inflammatory demyelinating lesions. The normal T2-weighted appearance of ongoing primary myelination in very young children may mimic the lesion appearance in children with a first attack of MS or children with acute disseminated encephalomyelitis (ADEM), in whom lesions are large, hazy, and ill defined.
- 2.
Dental hardware, such as braces and retainers, can cause significant susceptibility artifact. Sequences especially prone to artifact are diffusion-weighted (DW) imaging and spoiled gradient-recalled echo imaging.
- 3.
Tolerability of MR imaging scan length is a challenge in pediatrics, and often results in motion artifact–laden images. However, with the assistance of MR imaging–compatible movie goggles and headphones, children can tolerate lying still for longer durations of time. In very young children, sedation may be necessary to obtain sufficient imaging. Advances in MR imaging technology, such as parallel imaging, have significantly decreased scan time. However, situations may arise whereby a child has difficulty lying still for the total duration of the scan protocol; in these cases an abbreviated set of images without motion artifact is preferable to a complete protocol laden with motion artifact.
Introduction
Magnetic resonance (MR) imaging is perhaps one of the most important paraclinical tools for the diagnosis of multiple sclerosis (MS) and for monitoring disease progression and treatment response. However, the value of MR imaging in informing clinical management of MS patients largely depends on the use of a consistent and standard imaging protocol. The goals of MR imaging in MS include: confirmation of an MS diagnosis before a second clinical attack in individuals with an acute demyelinating syndrome according to dissemination in time and space criteria ; exclusion of alternative diagnoses ; and prediction of outcome. In addition, clinicians caring for patients with MS rely on comparison of serial scans to qualitatively evaluate the rate of new lesion accrual for diagnosis, to inform on treatment decisions, and to monitor disease evolution apparent by formation of confluent lesions and atrophy. A standard MR imaging acquisition protocol and reporting method will improve the accuracy and reliability of MR imaging evaluation among radiologists and clinicians within and across centers, and will aid the clinician in managing patients with MS.
An expert panel of adult MS radiologists and neurologists within the Consortium of MS Centers published a consensus-based MR imaging protocol for use in the investigation and monitoring of patients with MS. However, the panel indicated that the applicability of the protocol to pediatric-onset MS requires further study. Although many of the MR imaging features of MS in children may overlap with those of adults, specific considerations unique to the imaging of children and the distinct MR imaging features of MS in prepubertal children may necessitate a revised MR imaging protocol for pediatric MS.
The goal of this article is to provide clinicians and neuroradiologists caring for children with demyelinating disorders with a suggested standard MR imaging acquisition and reporting protocol, and to define a standard lexicon for lesion features typical of MS in children. Only conventional MR imaging sequences used in routine clinical practice and standard to most scanners are discussed; advanced MR imaging techniques are being increasingly applied in MS research, but are not yet applicable or feasible in the clinical setting. As considerable overlap exists between the features of pediatric-onset and adult-onset MS, these recommendations may be of relevance to adult clinicians and radiologists. The recommendations are based on recent MR imaging studies conducted in pediatric MS and on the expertise of pediatric MS neurologists and neuroradiologists at The Hospital for Sick Children (Toronto, Canada), the lead center for the Canadian Pediatric Demyelinating Disease Network, a prospective cohort study in which standardized clinical and MR imaging data and biological samples are collected at onset and serially in children with incident demyelination.
Unique considerations in MR imaging of children
There are several considerations to be mindful of when imaging the child being investigated for MS.
- 1.
In very young children, primary myelination is not yet complete. Use of T2-weighted sequences and, to a greater extent, fluid-attenuated inversion recovery (FLAIR) imaging, is not optimal for the assessment of the presence of inflammatory demyelinating lesions. The normal T2-weighted appearance of ongoing primary myelination in very young children may mimic the lesion appearance in children with a first attack of MS or children with acute disseminated encephalomyelitis (ADEM), in whom lesions are large, hazy, and ill defined.
- 2.
Dental hardware, such as braces and retainers, can cause significant susceptibility artifact. Sequences especially prone to artifact are diffusion-weighted (DW) imaging and spoiled gradient-recalled echo imaging.
- 3.
Tolerability of MR imaging scan length is a challenge in pediatrics, and often results in motion artifact–laden images. However, with the assistance of MR imaging–compatible movie goggles and headphones, children can tolerate lying still for longer durations of time. In very young children, sedation may be necessary to obtain sufficient imaging. Advances in MR imaging technology, such as parallel imaging, have significantly decreased scan time. However, situations may arise whereby a child has difficulty lying still for the total duration of the scan protocol; in these cases an abbreviated set of images without motion artifact is preferable to a complete protocol laden with motion artifact.
Standard MR imaging protocol for pediatric demyelination
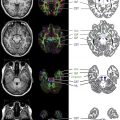
Table 1 describes the suggested standard protocol for pediatric demyelinating disorders, and indicates a recommended minimum set of scans for situations whereby a child cannot tolerate the complete protocol. Consistent prescription of slice angulation is important for evaluation of new lesion formation across serial scans; oblique axial images, where the acquisition plane is parallel to the subcallosal line joining the inferior margin of the genu and inferior margin of the splenium, are recommended. Contiguous slices of 3-mm thickness are recommended to permit accurate lesion detection, given that minimum MS lesion diameter criteria are approximately 3 mm. Attention should be given to ensure complete head coverage. To avoid distortions or edge blurring sometimes present in the first or last slice of a slab when the whole brain is not covered, acquisition of 1 to 2 slices of air outside the skull is recommended. This action is especially important when new lesions are present in regions of the brain omitted when whole brain coverage is not achieved, such as the juxtacortical region or brainstem ( Fig. 1 ), both of which are important components of the 2010 revisions to the McDonald criteria for dissemination in space.
| Order | Sequence | Recommendation | Comment |
|---|---|---|---|
| Brain Imaging | |||
| 1 | 3-plane localizer | Recommended | Prescribe oblique axial images a |
| 2 | 3D T1-weighted spoiled gradient-recalled echo imaging | Recommended | By increasing TR to 30 ms, contrast is comparable with the contrast on SE imaging; important in assessment of T1-hypointense lesion formation |
| 3 | Sagittal FLAIR | Recommended b | |
| 4 | Axial T2-weighted FSE or TSE | Recommended b | |
| 5 | Axial DWI | Recommended | In children presenting with acute symptoms, DWI is important to rule out arterial ischemic stroke |
| 6 | Contrast Administration | ||
| 7 | Axial FLAIR | Recommended | Acquired during 5-min delay between contrast injection and postcontrast imaging |
| 8 | Axial T1-weighted postcontrast spoiled gradient-recalled echo imaging | Recommended | By increasing the TR to 30 ms, the conspicuity of gadolinium enhancement is more comparable with that of SE imaging |
| Optional Orbital Imaging (Acquired Following Contrast Administration) | |||
| 1 | 3-plane localizer | If clinically indicated | Prescribe oblique axial images a |
| 2 | Coronal and axial T2-weighted fat-saturated imaging | If clinically indicated | Should include imaging of the optic nerves through to and including the optic chiasm |
| 3 | Coronal and axial T1-weighted postcontrast fat-saturated imaging | If clinically indicated | If acquired with brain protocol, acquire after sequence #8 under Brain Imaging |
| Optional Spinal Cord Imaging (Acquired Following Contrast Administration) | |||
| 1 | 3-plane localizer | If clinically indicated | |
| 2 | Sagittal T2-weighted FSE or TSE c | If clinically indicated | Acquire in superior, middle, and inferior sections |
| 3 | Axial T2-weighted FSE or TSE | If clinically indicated | Acquire only through regions of interest |
| 4 | Sagittal T1-weighted postcontrast SE | If clinically indicated | If acquired with the brain protocol, acquire after sequence #8 under Brain Imaging |
a Axial images parallel to the genu-splenium subcallosal line.
b May be omitted when child or adolescent cannot tolerate the scan.
c A short-tau inversion recovery (STIR) sequence may be acquired either in place of or in addition to T2-weighted FSE images to enhance ability to detect inconspicuous intramedullary lesions.
The rationale for the sequences included in Table 1 is summarized as follows:
- 1.
Three-dimensional (3D) T1-weighted imaging. A spoiled gradient-recalled echo sequence is recommended for its efficiency; that is, one can achieve a higher signal-to-noise ratio (SNR) with a shorter acquisition time compared with spin-echo (SE) imaging. 3D imaging also enables reformatting of the data into axial, coronal, or sagittal planes, and is often used in centers performing volumetric analyses. Acquiring T1-weighted imaging permits evaluation of black-hole accrual over time, the presence of which is highly predictive of MS in children with acute demyelination. Black holes initially were defined on SE imaging. However, increasing the repetition time (TR) of a T1-weighted 3D spoiled gradient-recalled echo sequence from the typical 20 milliseconds used for high-contrast, high-resolution applications to 30 milliseconds mutes the contrast to a level similar to that of an SE sequence. Lastly, T1-weighted imaging performed before contrast injection permits confirmation of lesion enhancement when present.
- 2.
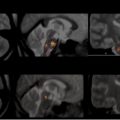
Axial T2-weighted imaging. Fast or turbo spin-echo imaging with an echo time (TE) between 80 and 120 milliseconds is recommended; a longer TE increases lesion conspicuity, at the cost of lower SNR. T2-weighted imaging provides better contrast than fluid-attenuated inversion recovery (FLAIR) for infratentorial lesion detection ( Fig. 2 ). Infratentorial lesions and, specifically, brainstem lesions are important features of MS in children. The presence of a “false lesion” in the pons caused by a deep interpeduncular cistern leading to a partial volume effect has been reported, and warrants consideration when interpreting pontine lesions. An intermediate-weighted or proton density is not thought to contribute to lesion detection beyond that of combined T2-weighted and FLAIR imaging, but may be used in some centers because it provides another contrast for automated lesion-detection techniques.
Fig. 2
Axial T2-weighted ( top row ) and axial fluid-attenuated inversion recovery (FLAIR) ( bottom row ) images of a child with multiple sclerosis. Two brainstem lesions ( arrows ) are visible on T2-weighted imaging in A-1, whereas both lesions ( arrows ) are more inconspicuous on FLAIR (A-2). At another level of the brainstem, the T2 lesion in B-1 ( arrow ) is not visible on FLAIR (B-2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree