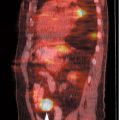
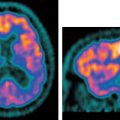
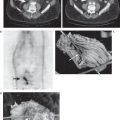
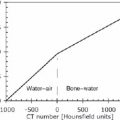
5 The standard uptake value (SUV) is also known as The SUV is a semiquantitative measurement. Numerous factors can result in erroneous results.2
Standardized Uptake Value
Eugene C. Lin, Abass Alavi, and Paul E. Kinahan
 SUV Calculation
SUV Calculation
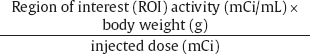
Pitfalls
Pearls
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree