Fig. 1
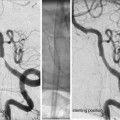
Complex aneurysms which are suboptimally addressed by conventional endovascular techniques. (a) Very large, wide-necked basilar trunk aneurysm. (b) Long segment fusiform, circumferential aneurysm of the internal carotid artery. (c) Giant recurrence of an aneurysm after conventional coil embolization. All three of these lesions are potentially amenable to reconstruction with flow-diverting constructs
1.
Very large or giant size
2.
Dysplastic or fusiform morphology
3.
Recurrence after prior surgical or endovascular treatment
4.
Other anatomical feature(s) making conventional surgical or endovascular treatment challenging or impossible
Flow-diverting stents may allow a constructive, technically feasible, definitive, and durable treatment option in many of these cases that were previously deemed “complex” or “untreatable” in the past.
Theoretical Background for Flow Diversion
Clinical Need for Flow Diverters
Aneurysms Amenable to Conventional Endovascular Treatment
Amenable Lesions
Small (<10 mm) cerebral aneurysms with (<4 mm) narrow necks are typically optimal for endovascular therapy.
Account for the majority of ruptured and many of the unruptured aneurysms encountered in clinical practice.
Conventional Endovascular (Endosaccular) Therapy
Endovascular treatment of aneurysms has been predicated upon filling the aneurysm sac with embolic material: most commonly platinum coils.
Clinical Evidence: Endovascular Coil
Embolization has been demonstrated to lead to favorable clinical outcomes for the majority of cerebral aneurysms.
When compared directly, outcomes with endovascular therapy are as good, if not better than surgery, for the majority of the ruptured and unruptured aneurysms that are encountered in clinical practice.
Complex Aneurysms: Often Difficult or Impossible to Treat with Conventional Endovascular Approaches
Large and Giant Aneurysms
Very high levels of incomplete occlusion (with estimates ranging between 50 and 85 %) and recurrence (40–70 %) and frequently require multiple treatments and re-treatments.
Treatments and re-treatments, potentially along with the progressive aneurysm growth, may result in significant cumulative morbidity and mortality. Jahromi et al. calculated rates of per treatment mortality and morbidity of 8 and 20 %, respectively, with an overall cumulative morbidity and mortality of 55 % for the endovascular treatment of giant aneurysms.
Failed Prior Treatment: Aneurysms Which Fail Initial Endovascular Treatment, Regardless of Size, Also Represent a Challenging Subtype of Lesions
Approximately 50 % of re-treated aneurysms demonstrate another recurrence after re-treatment.
Fusiform, Circumferential, and Very Wide-Necked Aneurysms
Occurrence: Rare and highly variable group of lesions. b. Treatment: Conventional treatment of these lesions is associated with a relatively high rate of periprocedural and intra-procedural complications, particularly when a reconstructive approach (rather than a vessel occlusion) is required. Oftentimes, when a conventional constructive approach is taken, the result is temporary, and the lesions continue to recur locally and enlarge.
Complex Aneurysms: Reasons for Failure with Conventional Endovascular Therapy
These complex aneurysms often fail conventional endovascular treatment for two main reasons:
Low Packing Density
For small, narrow-necked aneurysms, packing densities in the range of 40 % are possible, and at this level of aneurysm occlusion, recanalization is uncommon.
For larger and giant aneurysms, packing densities are more commonly in the range of 20 %, which may not provide the structural integrity to support a durable aneurysm occlusion.
This is a particular problem if the aneurysm contains a large volume of soft, intraluminal thrombus, as with time, the coil mass can become compacted into the thrombus, leading to relatively rapid and large recanalizations (Fig. 1).
Inadequate Reconstruction of the Aneurysm-Parent Vessel Interface
When the aneurysm neck comprises a significant length and circumference of the parent artery, it is very difficult to reconstruct a homogenous, smooth, and continuous surface by packing the aneurysm with embolization coils (even with the use of an adjunctive device such as a balloon or conventional coil-assist stent).
The interface is usually irregular with large interstices between coil loops in the region of the aneurysm neck.
These gaps allow continued flow into the aneurysm.
This incomplete neck reconstruction leaves the aneurysm susceptible to recanalization.
Flow Diversion: Mechanistic Basis
Concept
Flow diversion differs from conventional endovascular, endosaccular aneurysm treatment.
In contrast to endosaccular treatment, flow diversion is a primarily endoluminal approach to aneurysm treatment.
Device Characteristics
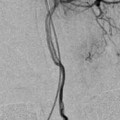
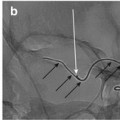
Flow diverters are typically braided stent-like constructs with low pore density and high metal surface area (Fig. 2).

Fig. 2
Pipeline Embolization Device (PED): The PED is a flexible, braided, self-expanding microstent, which is delivered through a microcatheter with a 0.027″ internal diameter. When deployed, the device provides approximately 30–35 % metal surface area coverage over the reconstructed segment
The devices are deployed through a microcatheter and positioned to completely bridge the aneurysm neck, spanning from normal vessel proximally to normal vessel distally.
While aneurysms fill solely due to the trajectory of inflow and outflow vectors, regional branches fill on the basis of an arterial to venous pressure drop.
Pressure gradients drive flow through the branches and support their patency despite substantial degrees of metal coverage over their ostia.
Optimally designed flow-diverting constructs are of a porosity that efficiently induces aneurysm occlusion while at the same time reliably allowing the continued patency of regional eloquent branches which are also covered by the construct.
The operator to some degree can customize the degree of metal surface area coverage during the procedure by applying variable load to the microcatheter during deployment, compressing the braid over segments of the parent artery.
Process of Reconstruction
Reconstruction of the parent artery with aneurysm occlusion occurs over a period of days to months through the following sequence:
Mechanical: Flow Disruption (Immediate)
The construct disrupts both the inflow and outflow of blood within the aneurysm, redirecting the primary vector of blood flow along the course of the anatomically reformed parent artery.
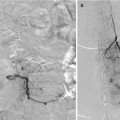
While the aneurysm may still fill with contrast, the intra-aneurysmal flow is disrupted and shear forces upon the aneurysm wall are reduced (Fig. 3).
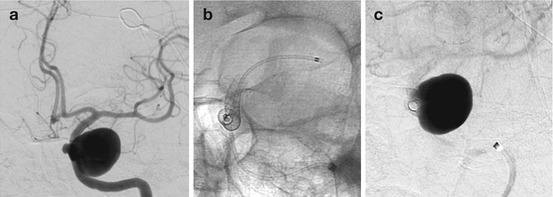
Fig. 3
Progression of curative reconstruction after successful flow diversion: mechanical reconstruction. Subtracted angiogram in the PA projection demonstrates a giant aneurysm involving the cavernous segment of the left internal carotid artery (a). Native image in the same projection depicts a construct composed of three pipeline devices reconstructing the left internal carotid artery (b). Subtracted image in the capillary phase of angiography demonstrating the hemodynamic effects of mechanical reconstruction with persistent stasis of contrast within the aneurysm (c)
Physiological: Aneurysm Thrombosis (Days to Weeks)
The intra-aneurysmal flow disruption creates an environment conducive to progressive thrombosis.
When this process is complete, angiographic imaging demonstrates complete occlusion.
Cross-sectional imaging demonstrates a thrombus mass within the aneurysm.
The rate of this thrombosis seems to vary significantly with aneurysm size, location, and the degree of neck coverage.
Clinically, local mass effect and/or transmural inflammatory changes secondary to the thrombosis may result in an exacerbation of the originally present clinical symptoms or, in some cases, new symptoms (e.g., headache or cranial neuropathy).
In some cases, these new symptoms can be treated with steroids (Fig. 4).
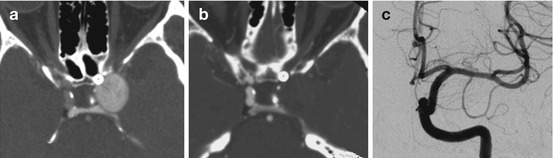
Fig. 4
Progression of curative reconstruction after successful flow diversion: physiological reconstruction. Computed tomographic angiographic image in the axial plane 24 h after reconstruction of the aneurysm depicted in Fig. 2 demonstrates the construct in position with continued filling of the aneurysm with contrast (a). Follow-up CTA 2 weeks later demonstrates complete thrombosis of the aneurysm (b). Angiographic follow-up performed 6 months after treatment confirms complete occlusion of the aneurysm with anatomical remodeling of the parent artery (c)
Biological: Construct Endothelialization and Thrombus Resorption (Months)
When the aneurysm is completely occluded, the construct may become endothelialized.
This forms a permanent biological seal across the aneurysm-parent artery interface, to functionally bridge the normal proximal and distal parent arterial segments.
When the aneurysm is completely excluded from the circulation, the intra-aneurysmal thrombus begins to resorb and the entire aneurysm mass collapses around the periphery of the construct (Fig. 5).
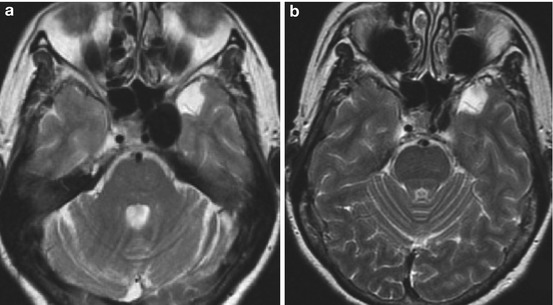
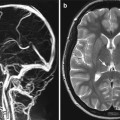
Fig. 5
Progression of curative reconstruction after successful flow diversion: anatomical restoration. Long TR-weighted sequence in the axial plane demonstrates the left cavernous segment aneurysm as a hypointense mass with some regional mass effect (a). Follow-up MR performed at 6 months demonstrates near-complete resolution of mass effect after curative reconstruction and resorption of the intra-aneurysmal thrombus mass (b)
Symptoms related to aneurysm mass effect or pulsation may resolve at this point (to the extent that they are reversible).
Clinical Applications of the Pipeline Embolization Device: Available Data
Available Devices
Pipeline Embolization Device (PED; Covidien/ev3, Mansfield, MA, USA)
The PED is the only flow diverter that has been cleared by the US Food and Drug Administration and has CE Mark.
Commercially available in the USA and worldwide.
Silk+ (Balt Extrusion, Montmorency, France)
CE Mark in Europe with clinical application outside of the USA
Surpass (Surpass Medical Ltd., Tel-Aviv, Israel)
CE Mark in Europe with clinical application outside of the USA
Pipeline Embolization Device: Clinical Experience
Compassionate Use
Initial cases performed with the PED predated its regulatory approval.
Cases most often performed within the context of “compassionate use”: Case-specific permission was obtained from regulatory bodies for patients with aneurysms not treatable with conventional surgical or endovascular approaches.
These cases provided the first “proof of concept” that flow diversion could be successfully and safely applied in cases which lacked other options.