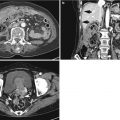
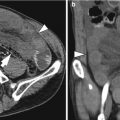
Histological layers
1st: superficial mucosa
2nd: deep mucosa (muscularis mucosa)
3rd: submucosa
4th: muscularis propria
5th: serosa
Neoplastic lesions
GIST
O
O
O
Leiomyoma/leiomyosarcoma
O
(O)
O
Lipoma
O
Carcinoid
O
O
O
Granular cell tumor
O
O
Glomus tumor
(O)
O
Schwannoma
O
Metastasis
O
O
O
O
O
Nonneoplastic lesions
Ectopic pancreas
O
(O)
(O)
Gastritis cystica profunda
O
Brunner’s gland hamartoma
O
Table 7.2
CT differential points among gastric subepithelial lesions
GIST | Leiomyoma | Schwannoma | Ectopic pancreas | Glomus tumor | |
|---|---|---|---|---|---|
Sex | M ≥ F | M ≈ F | M ≈ F | M ≈ F | F ≫ M |
Location | Even distribution | Cardia | Even distribution | Antrum, pylorus | Exclusively antrum |
Shape (LD/SD ratio) | Round or slightly oval | Oval or flat (LD/SD ratio >1.4) | Round or slightly oval | ||
Degree of lesion enhancement, homogeneity | Variable | Low or iso, homogeneous | Low, homogeneous | Variable | Strong (lesion-to-aorta ratio on portal phase >0.86) hemangioma-like enhancement |
Enlarged lymph nodes | − (+ with intratumoral necrosis) | − | ++ (especially when tumors ≥5 cm) | − | − |
Overlying mucosa | Thin, iso-attenuating to adjacent mucosa | Strong enhancement | Thick (>2.6 mm) | ||
7.1 Gastrointestinal Stromal Tumor (GIST)
GIST occurs at every level of the tubular gastrointestinal tract and rarely occurs at the omentum and mesentery. The most common site of GIST is the stomach (60 ~ 70 %), followed by small bowel (20 ~ 30 %), colorectum (<10 %), and esophagus (<5 %) (Fenoglio-Preiser et al. 2000). GIST is the most common mesenchymal tumor of the stomach and accounts for 2.2 % of malignant gastric tumors. All GISTs are potentially malignant and, therefore, cannot be classified as benign versus malignant. Instead, the risk of an aggressive clinical behavior of gastric GISTs is stratified based on tumor size and mitotic count (Fletcher et al. 2002). Microscopically, GISTs typically have spindle cell morphology, but epithelioid morphology may be seen. Immunohistochemically, CD117 (c-kit) protein is a specific marker for GISTs with 95 % positivity for the protein (Miettinen and Lasota 2001).
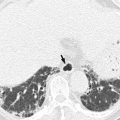
On barium study, small GISTs commonly present with smooth borders forming right or obtuse angles with the nearby gastric wall. The mucosal surface is usually intact except for areas of ulceration, which are generally present in 50 % of GISTs. Ulcerations fill with barium causing a bull’s eye or target appearance. As the tumor grows, it may project outside the stomach, showing an exophytic or mixed growth pattern. If the tumor outstrips its blood supply, it can necrose internally, creating a central fluid-filled cavity with hemorrhage and cavitation that can eventually communicate into the lumen of the bowel (Gastrointestinal stromal tumor 2013). The presence of bridging fold or slow fade-out of gastric folds at both ends of the mass is known to be one of several characteristics that may permit correct differentiation of a subepithelial tumor from a mucosal lesion. However, barium studies are limited to evaluate exophytic masses, and in staging and detection of small or exophytic GISTs, this method is of little value. On CT, small GISTs are seen as smooth, sharply demarcated subepithelial mass with an intact overlying mucosal layer and with homogeneous attenuation. Large GISTs appear as heterogeneous masses with a low-attenuating focus due to areas of hemorrhage or necrosis.
Malignancy is characterized by local invasion or distant metastasis, usually to the liver, omentum, and peritoneum. Contrary to adenocarcinoma or lymphoma, lymph node metastasis is uncommon (<5 %) and therefore, imaging usually shows absence of lymph node enlargement. If metastases are not present, other CT or MRI features suggesting malignancy include size (>5 cm), heterogeneous enhancement, and necrosis (Kim et al. 2004). The absence of lymphadenopathy and the presence of necrosis can be used as differential point of GIST from gastric schwannoma which is the second most common mesenchymal tumor of the stomach (Choi et al. 2012).
7.2 Schwannoma
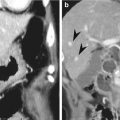
Gastrointestinal schwannoma is a rare mesenchymal tumor and it consists of 3 % of all gastrointestinal mesenchymal tumors. The ratio of incidence between GIST and schwannoma is reported to be 8 ~ 14:1 (Voltaggio et al. 2012). Gastric schwannoma differs histologically from soft tissue schwannoma in several ways. Gastric schwannomas lack encapsulation as a result of their origin from the dispersed autonomic nerve Schwann cells, as opposed to being encased by epineurium, as seen in soft tissue schwannomas. In addition, nuclear palisading, xanthoma cells, and vascular hyalinization and dilation, which are commonly seen in soft tissue schwannomas, are rare in gastric schwannoma. On the other hand, prominent lymphoid infiltration and peripheral lymphoid cuffing are the unique histological features of gastric schwannoma. S-100 protein positivity is a unique IHC feature of gastric schwannoma (Voltaggio et al. 2012).
On CT, gastric schwannoma usually appears as a homogeneous, iso- or low-attenuating subepithelial mass with intact or ulcerated overlying mucosa. On the contrary to GISTs, intratumoral necrosis is rare even in large gastric schwannomas. The presence of enlarged lymph nodes at perigastric or distant areas is known to be a differential point from GIST in which lymphadenopathy is rare (Choi et al. 2012).
7.3 Leiomyoma and Leiomyosarcoma
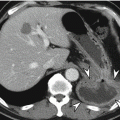
Leiomyoma or leiomyosarcoma is a true smooth muscle tumor which can be differentiated from GISTs or other mesenchymal tumors by their positive immunoreactivity for desmin and smooth muscle actin. Leiomyoma occurs frequently in the esophagus or colorectum, but rarely in the stomach. Gastric leiomyoma is mainly located at gastric cardia and appears as a homogeneous low-attenuating subepithelial lesion on CT (Lee et al. 2007). Surface ulceration or calcification is rarely found.
7.4 Lipoma
Lipomas composed of mature adipose tissue may be observed in the stomach. They typically protrude into the lumen and appear as a compressible, round, well-circumscribed subepithelial nodule on endoscopy. On barium study, it appears as a well-demarcated nodule with intact overlying layer. Easy compressibility and change in shape during peristaltic movement can be a suggestive feature of gastric lipoma. On CT, typical fat attenuation of the tumor makes the diagnosis of a lipoma easy.
7.5 Glomus Tumor
Glomus tumors originate from modified smooth muscle cells of perivascular glomus bodies, which are specialized arteriovenous communications that regulate skin temperature (Hur et al. 2011). Although the most common location of glomus tumor is the subungual region, this tumor can occur elsewhere in the body. Gastrointestinal glomus tumor is very rare with a female predominance and consists of <1 % of all gastrointestinal subepithelial tumor. Gastric glomus tumor is usually located at gastric antrum and shows surface ulceration as the tumor enlarges (Hur et al. 2011). Immunohistochemically, it shows positivity for smooth muscle actin and synaptophysin and negativity for chromogranin A and c-kit.
On CT, predominant antral location, hemangioma-like enhancement pattern on dynamic imaging, strong and persistent enhancement on portal phase, and thick overlying mucosal layer can be used as differential features of glomus tumor from other gastric subepithelial tumors (Hur et al. 2011).
7.6 Nonneoplastic Subepithelial Lesions
Various kinds of nonneoplastic lesions may manifest themselves as a subepithelial lesion and thus easily mimic tumorous conditions. They include ectopic pancreas, gastritis cystica profunda, and amyloidoma. Ectopic pancreas, also referred to as heterotopic, accessory, or aberrant pancreas, is defined as pancreatic tissue lacking anatomic and vascular continuity with the main body of the gland; an ectopic pancreas is usually located in the stomach, duodenum, or jejunum (Kim et al. 2009). A combination of five CT findings such as prominent enhancement of the overlying mucosa, antral or duodenal location, large long diameter/short diameter ratio, endophytic growth pattern, and ill-defined lesion border may be helpful for the differentiation from subepithelial tumors including GIST and leiomyoma (Kim et al. 2009). Gastritis cystica profunda is an ectopic proliferation of gastric glandular elements, typically located beneath a normal mucosal layer. Gastritis cystica profunda is a rare submucosal lesion that is often reported in the setting of a prior gastroenterostomy. However, it can occasionally be found in the stomach without previous surgery. Histologically, the lesion extends into the gastric submucosal layer and is composed of elongated hyperplastic gastric foveolae with hyperplasia and cystic dilation of the gastric glands. This lesion presents as giant gastric folds, a submucosal tumor, or an isolated polyp (gastritis cystica polyposa). CT findings of gastritis cystica profunda may vary from a homogeneously enhancing polypoid lesion all the way to a purely cystic subepithelial lesion.
Summary
1.
Various kinds of neoplastic and nonneoplastic lesions appear as a subepithelial lesion in the stomach.
2.
Most common subepithelial tumor in the stomach is a gastrointestinal stromal tumor (GIST).
3.
Most common site of GIST is the stomach, followed by the small bowel, colorectum, esophagus, and omentum or mesentery.
4.
The risk of an aggressive clinical behavior of gastric GISTs is stratified based on tumor size and mitotic count.
5.
CT or MRI features of GIST suggesting malignancy include large size (>5 cm), heterogeneous enhancement, and intratumoral necrosis.
6.
The presence of enlarged lymph nodes at perigastric or distant areas and the absence of intratumoral necrosis are known to be differential points of gastric schwannoma from GISTs.
7.
Gastric leiomyoma is mainly located at gastric cardia and appears as a homogeneous low-attenuating subepithelial lesion on CT.
8.
Predominant antral location, hemangioma-like enhancement pattern on dynamic imaging, strong and persistent enhancement on portal phase, and thick overlying mucosal layer on CT can be used as differential CT features of glomus tumor from other gastric subepithelial tumors.
9.
A combination of five CT findings such as prominent enhancement of the overlying mucosa, antral or duodenal location, large long diameter/short diameter ratio, endophytic growth pattern, and ill-defined lesion border may be helpful for the differentiation of ectopic pancreas from subepithelial tumors including GIST and leiomyoma.
7.7 Illustrations Gastric Subepithelial Lesions: Subepithelial Lesions of the Stomach
7.7.1 Correlation of Findings Between Endoscopic Ultrasonography (EUS) and Histopathology with Schematic Diagram of Normal Gastric Wall
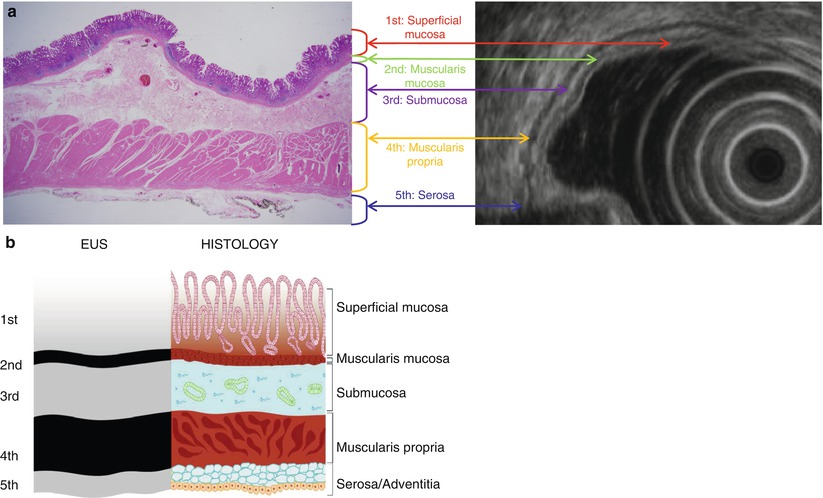
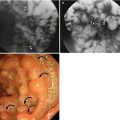
Fig. 7.1
Correlation of findings between endoscopic ultrasonography (EUS) and histopathology with schematic diagram of normal gastric wall. (a) Real histopathology and EUS images. (b) Corresponding schematic diagram. On EUS, gastric layer consists of five layers of alternating echogenicity: (1) innermost hyperechoic layer representing lumen-mucosa interface and superficial mucosa on histopathology, (2) hypoechoic layer indicating muscularis mucosa, (3) hyperechoic layer for submucosa, (4) hypoechoic layer indicating muscularis propria, and (5) outermost hyperechoic layer for serosa
7.7.2 Schematic Diagrams Showing Predominant Location of Gastric Subepithelial Lesions
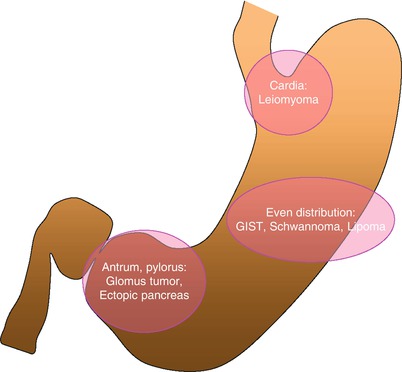
Fig. 7.2




Schematic diagram showing a predominant location of gastric subepithelial lesions. Gastric leiomyoma is usually located at gastric cardia. Gastric antrum and pylorus are the common sites for glomus tumor and ectopic pancreas
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree