(1)
Cleveland Clinic, Emeritus Staff, Cleveland, OH, USA
Keywords
PET radiopharmaceuticalsSynthesisSynthesis boxQuality controlIntroduction
PET radiopharmaceuticals are uniquely different from SPECT radiopharmaceuticals in that the former have radionuclides that are positron emitters and the majority of them have short physical half-lives. The most common PET radionuclides are 11C, 15O, 13N, 18F, and 82Rb, which are short-lived (see Table 7.2) and put limitations on the synthesis time for PET radiopharmaceuticals and their clinical use. The attractive advantage of PET radiopharmaceuticals, however, is that the ligands used in radiopharmaceuticals are common analogs of biological molecules and, therefore, often depict a true representation of biological processes after in vivo administration. For example, 18F-fluorodeoxyglucose (FDG) is an analog of glucose used for cellular metabolism and H215O for cerebral perfusion.
Automated Synthesis Device
Conventional manual methods of synthesis of radiopharmaceuticals using a high level of radioactivity are likely to subject the personnel involved in the synthesis to high radiation exposure. This is particularly true with short-lived positron emitters such as 11C, 13N, 15O, and 18F, because the quantity of these radionuclides handled in the synthesis is very high. To minimize the level of exposure, automated modules have been devised for the synthesis of PET radiopharmaceuticals.
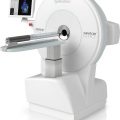
The automated synthesis device, often called the black box, is a unit controlled by microprocessors and software programs to carry out the sequential physical and chemical steps to accomplish the entire synthesis of a radiolabeled product. The unit consists of templates or vials prefilled with required chemicals attached to the apparatus via tubings that are connected to solenoid valves to switch on and off as needed. Most black boxes are small enough to be placed in a space of 20 × 20 × 20 in. and are capable of self-cleaning. In some units, disposable cassettes (cartridges) are employed so that new cassettes can be used for each new synthesis, thus minimizing contamination and radiation exposure. Various parameters for synthesis such as time, pressure, volume, and other requisites are all controlled by a remote computer using appropriate software. The unit has a graphic display showing the status of the ongoing process. After the synthesis, a report with the date and start and end time of the radiosynthesis and the calculated yield is printed out. Technologists can operate these units very easily. Automated synthesis modules for 18F-FDG, 13N-NH3, 11C-CH3I, 11C-HCN, 11C-acetate, and a few other PET tracers are commercially available. Versatile automated modules are commercially available to use for the synthesis of a variety of PET tracers in the single module. This is accomplished by simple exchange or modification of various segments inside the unit to suit the specific product synthesis. To minimize radiation exposure, often the synthesis box is placed inside a minicell (see Chap. 7). After each synthesis, the product is passed through a high-performance liquid chromatography (HPLC) described later to achieve a high-purity finished product. A schematic diagram of a black box for 18F-FDG synthesis is shown in Fig. 8.1. Often the synthesis box is placed inside a minicell to minimize radiation exposure. An automated multi-synthesis module (FASTlab2) for the synthesis of different PET radiopharmaceuticals marketed by GE Healthcare is shown in Fig. 8.2. Other vendors include Siemens Medical Solutions, Inc. (Explora), IBA (Synthera), and Eckert & Ziegler (FDG-Plus).
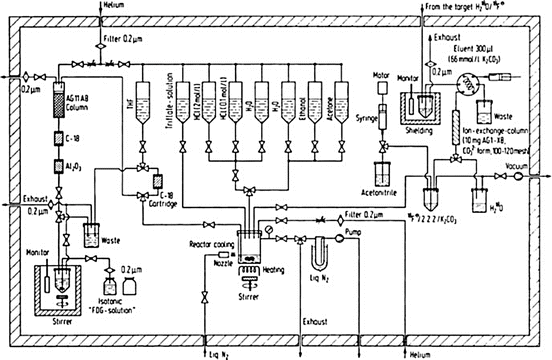


Fig. 8.1
A schematic block diagram showing different components in the 18F-FDG synthesis box (Reproduced with kind permission of Kluwer Academic Publishers from Crouzel C et al. (1993) Radiochemistry automation PET. In: Stöcklin G, Pike VW (eds) Radiopharmaceuticals for positron emission tomography, Kluwer Academic, Dordrecht, the Netherlands, p 64. Fig. 9)

Fig. 8.2
Automated synthesis box, FASTlab2, from GE Healthcare (Courtesy of GE Healthcare)
PET Radiopharmaceuticals
Many radiopharmaceuticals have been used for PET imaging; however, only a few are routinely utilized for clinical purposes. Almost all of them are labeled with one of the four common positron emitters: 11C, 13N, 15O, and 18F. Of the four, 18F is preferred most, since it has a relatively longer half-life (t 1/2 = 110 min) that allows its supply to relatively remote places. In all cases, a suitable synthesis method is adopted to provide a stable product with good labeling yield, high specific activity, high purity, and, most importantly, high in vivo tissue selectivity. The following is a description of the syntheses of the common clinically used PET radiopharmaceuticals and a few with potential for future use.
18F-Sodium Fluoride
Fluorine-18 (t 1/2 = 110 min) is produced by irradiation of 18O-water with 10–18 MeV protons in a cyclotron and recovered as 18F-sodium fluoride by passing the irradiated water target mixture through a carbonate-type anion-exchange resin column. The water is forced out of the column with neon gas, whereas 18F− is retained on the column, which is recovered by elution with potassium carbonate solution. Its pH should be between 4.5 and 8.0. While 18F-sodium fluoride is most commonly used for the synthesis of FDG, it is also used for other 18F-labeled PET radiopharmaceuticals.
The US FDA has approved it for bone scintigraphy, since it localizes in bone by exchanging with 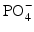 ion in the hydroxyapatite crystal.
ion in the hydroxyapatite crystal.
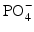 ion in the hydroxyapatite crystal.
ion in the hydroxyapatite crystal.18F-Fluorodeoxyglucose
18F-2-fluoro-2-deoxyglucose (2-FDG) is normally produced in places where a cyclotron is locally available. Its molecular formula is C8H11 18FO5 with molecular weight of 181.3 Da. 18F-2-FDG can be produced by electrophilic substitution with 18F-fluorine gas or nucleophilic displacement with 18F-fluoride ions. The radiochemical yield is low with the electrophilic substitution, so the nucleophilic displacement reaction has become the method of choice for 18F-FDG synthesis. Deoxyglucose is labeled with 18F– by nucleophilic displacement reaction of an acetylated sugar derivative followed by hydrolysis (Hamacher et al. 1986). In nucleophilic substitution, a fluoride ion reacts to fluorinate the sugar derivative. A solution of 1,3,4,6-tetra-O-acetyl-2-O-trifluoromethane-sulfonyl-β–d-mannopyranose in anhydrous acetonitrile is added to a dry residue of 18F-fluoride containing aminopolyether (Kryptofix 2.2.2) and potassium carbonate (Fig. 8.3). Kryptofix 2.2.2 is used as a catalyst to enhance the reactivity of the fluoride ions. The mixture is heated under reflux for about 5 min. The solution is then passed through a C-18 Sep-Pak column, and acetylated carbohydrates are eluted with tetrahydrofuran (THF), which are then hydrolyzed by refluxing in hydrochloric acid at 130 °C for 15 min. 18F-2-fluoro-2-deoxyglucose (2-FDG) is obtained by passing the hydrolysate through a C-18 Sep-Pak column. The yield can be as high as 60%, and the preparation time is approximately 50 min. The final solution is filtered through a 0.22-μm filter and diluted with saline, as needed. According to USP specifications, it should have pH of 4.5–7.5 and a specific activity of more than 1 Ci (37 GBq)/μmol. The chemical purity is limited to 50 μg/mL of Kryptofix 2.2.2 and 1 mg of 2-chloro-2-deoxy-d-glucose per total volume. Radiochemical purity should be >90%, as determined by the TLC method using activated silica gel as the solid phase and a mixture of acetonitrile and water (95:5) as the liquid phase.
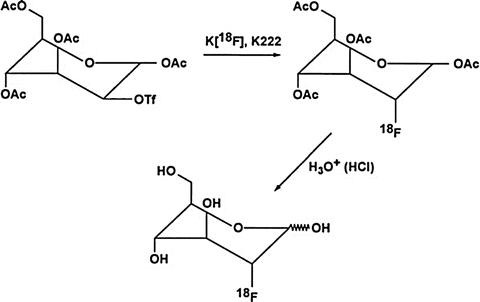

Fig. 8.3
Schematic synthesis of 18F-2-fluoro-2-deoxyglucose (FDG) (Reprinted with the permission of the Cleveland Clinic Center for Medical Art and Photography ©2009. All rights reserved)
Since Kryptofix 2.2.2 is toxic causing apnea and convulsions, modifications have been made to substitute it with tetrabutylammonium hydroxide or bicarbonate, which have been adopted by many commercial vendors. Also, in some other methods, the C-18 Sep-Pak column separation has been eliminated so as to carry out the acidic hydrolysis in the same vessel. In methods where Kryptofix 2.2.2 is still used, several Sep-Pak columns are used to separate Kryptofix 2.2.2 and reduce it to practically a negligible quantity.
The FDA has approved 18F-2-FDG for many clinical uses such as the metabolism in the brain and heart and the detection of epilepsy and various tumors. In metabolism, 18F-2-FDG is phosphorylated by hexokinase to 2-FDG-6-phosphate which is not metabolized further. It should be noted that 3-fluorodeoxyglucose (3-FDG) is not phosphorylated and hence is not trapped and essentially eliminated rapidly from the cell. This is why 3-FDG is not used for metabolic studies. Detailed protocols of 18F-FDG usage in humans are given in Chap. 13.
Because of the relatively longer half-life of 18F among the PET radionuclides, commercial and institutional facilities having cyclotrons produce 18F-FDG in bulk quantities and supply to nearby clinics and hospitals as needed. Supply can be made as far as 200 miles away with a loss of activity, which can be compensated by adding more activity. The details of 18F-FDG distribution is given in Chap. 10.
6-18F-l-Fluorodopa
Like 18F-2-FDG, 6-18F-l-fluorodopa is also produced in places where a cyclotron is available locally. There are several methods of synthesizing 6-18F-fluoro-3,4-dihydroxyphenylalanine (6-18F-l-fluorodopa), of which the method of fluorodemetallation using electrophilic fluorinating agents is most widely used. Electrophilic reactions involve the reaction of fluorine in the form of F+ with other molecules. Only the l-isomer of dopa is important, because the enzymes that convert dopa to dopamine, which is targeted by the radiopharmaceutical, are selective for this isomer. Initially, a suitably protected organomercury precursor (N-[trifluoroacetyl]-3,4-dimethoxy-6-trifluoroacetoxymercuriophenylalanine ethyl ester) of dopa is prepared. [18F]-labeled acetylhypofluorite prepared in the gas phase is then allowed to react with the mercury precursor in chloroform or acetonitrile at room temperature. Other precursors using metals such as tin, silicon, selenium, and germanium have been reported. Acid hydrolysis with 47% HBr provides a relatively high yield (10–12%) of 6-18F-l-fluorodopa (Luxen et al. 1992) compared with other available methods. Substitution at position 6 is most desirable, because this does not alter the behavior of dopa, whereas substitutions at 2 and 5 do. It is sterilized by filtering through a 0.22-μm membrane filter and is supplied at pH between 6 and 7. Normally EDTA and ascorbic acid are added to the final preparation for stability. Its specific activity should be more than 100 mCi (3.7 GBq)/mmol and radiochemical purity >95% as determined by HPLC. Mercury is the major chemical impurity (toxic) that originates from organomercuric precursor used in the synthesis, and its USP limit is 0.5 μg/mL of l-dopa solution. The molecular structure of 6-18F-l-fluorodopa is shown in Fig. 8.4a.
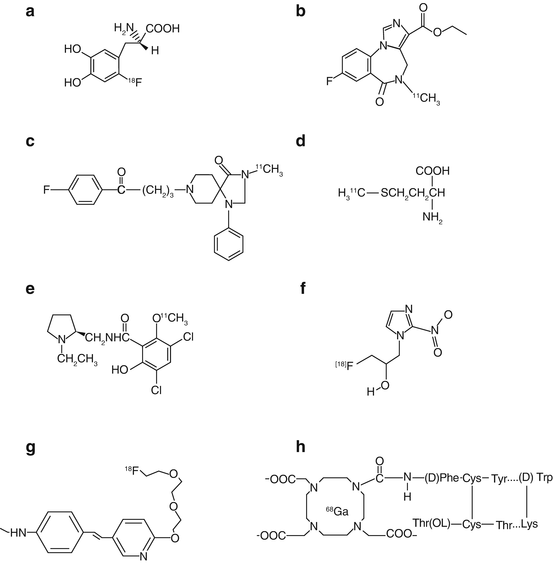

Fig. 8.4
Molecular structures of (a) 6-18F-l-fluorodopa, (b) 11C-flumazenil, (c) 11C-methylspiperone, (d) 11C-l-methionine, (e) 11C-raclopride, (f) 18F-fluoromisonidazole, (g) 18F-florbetapir, and (h) 68Ga-DOTATOC (Reprinted with the permission of the Cleveland Clinic Center for Medical Art and Photography©2009. All rights reserved)
It is particularly useful for the detection of Parkinson’s disease.
18F-Fluorothymidine
18F-Fluorothymidine (FLT) is prepared by nucleophilic reaction between 18F-sodium fluoride and a precursor, 2, 3′-anhydro-5′-O-benzoyl-2′-deoxythymidine, which is prepared by standard organic synthesis (Machula et al. 2000). 18F-Sodium fluoride is added to a mixture of Kryptofix 2.2.2 and potassium carbonate in acetonitrile, and the mixture is dried to a residue by heating at 120 °C for 5 min. The precursor in dimethyl sulfoxide (DMSO) is added to the dried residue and heated at 160°C for 10 min. Hydrolysis of the 5′-0-protecting group is performed with sodium hydroxide. 18F-FLT is isolated by passing through alumina Sep-Pak and further purified by using high-performance liquid chromatography (HPLC). The overall yield is about 45% and the radiochemical purity is more than 95%. The synthesis time is about 60 min.
Since thymidine is incorporated into DNA and provides a measure of cell proliferation, 18F-FLT is commonly used for in vivo diagnosis and characterization of tumors in humans.
18F-O-(2-Fluoroethyl)-l-Tyrosine
The synthesis of 18F-O-(2-fluoroethyl)-l-tyrosine (FET) is carried out in two steps (Wester et al. 1999): First, ethylene glycol-1,2-ditosylate in acetonitrile is reacted with dry 18F-containing Kryptofix 2.2.2 and potassium bicarbonate at 90°C for 10 min. The product is purified by absorbing it on a polystyrene cartridge and then eluting with dimethyl sulfoxide. Second, the eluate is mixed with dipotassium sodium salt of l-tyrosine and heated at 90°C for 10 min. The mixture is purified by HPLC and cation exchange to obtain 18F-FET. The radiochemical yield is about 40–45% with purity between 97 and 99%.
18F-FET is used as a PET tracer for the detection of a variety of tumors.
18F-Fluoromisonidazole
18F-Fluoromisonidazole (FMISO) is synthesized in one-step reaction between the protected precursor, 1-(2′-nitro-1′-imidazolyl)-2-O-tetrahydropyranyl-3-O-toluenesulfonyl-propanediol (NITTP) and 18F-containing Kryptofix 2.2.2 in acetonitrile solution (Kamarainen et al. 2004). The labeled product is hydrolyzed with acid to give 18F-FMISO, which is further purified by column chromatography using a Sep-Pak cartridge. From automated synthesis, the radiochemical yield is 34% at EOS after a synthesis time of 50 min. HPLC shows a radiochemical purity of 97%. The molecular structure of 18F-FMISO is shown in Fig. 8.4f.
18F-FMISO is a specific tracer used for detection of hypoxic tissues by PET.
18F-1-(5-Fluoro-5-Deoxy-α-Arabinofuranosyl)-2-Nitroimidazole
18F-1-(5-Fluoro-5-deoxy-α-arabinofuranosyl)-2-nitroimidazole (FAZA) is synthesized by nucleophilic substitution reaction between the precursor 1-(2,3-di-O-acetyl-5-O-tosyl-α–d-arabinofuranosyl)-2-nitroimidazole in DMSO solution and 18F-fluoride containing mixture of Kryptofix 2.2.2 and potassium carbonate at 100°C for 5 min (Reischl et al. 2005). The product is hydrolyzed with NaOH solution at 20°C for 2 min, and the solution is neutralized with NaH2PO4. It is further purified by HPLC and sterilized by 0.22-μm filtration. The radiochemical yield is about 61%.
18F-FAZA is used for the detection of tissue hypoxia by PET.
18F-Florbetapir
18F-Florbetapir (brand name 18F-AV-45 or Amyvid) has the molecular structure of (E)-4-(2-(6-(2-(2-(2-([18F]-fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N-methylbenzenamine. It is prepared by nucleophilic substitution reaction between 18F-containing mixture of Kryptofix 2.2.2 and potassium carbonate in acetonitrile solution and the precursor (E)-2-(2-(2-(2-tosyloxyethoxy)ethoxy)ethoxy)-5-(4-(tertbutoxycarbonyl (methyl)amino)styryl)pyridine (-OTs derivative) in DMSO solution at 115 °C for 10 min (Liu et al. 2010). The product is hydrolyzed by hydrochloric acid solution followed by neutralization with a solution of NaOH and ammonium acetate solution. The solution is loaded on a Sep-Pak C18 column, and unreacted 18F− is removed by rinsing with deionized water, and 18F-florbetapir is eluted with acetonitrile. 18F-Florbetapir is purified by HPLC and finally taken up in ethyl alcohol for clinical use. The radiochemical yield is 34% and the radiochemical purity is nearly 95%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree