ARTERIOVENOUS FISTULA
= AVF
= abnormal communication between artery + vein resulting in tremendous amount of flow due to high pressure gradient → enlargement + elongation of draining veins; NO nidus
Cause:
(1) Vessel laceration (delay between trauma + clinical manifestation ← delayed lysis of hematoma surrounding arterial laceration)
(2) Angiodysplasia: fibromuscular disease, neurofibromatosis, Ehlers-Danlos syndrome
(3) Congenital arteriovenous fistula
Location:
(a) carotid-cavernous sinus fistula (most common)
(b) vertebral artery fistula
(c) external carotid artery fistula (rare)
Carotid-Cavernous Sinus Fistula
= abnormal communication between veins of cavernous sinus and ≥ 1 branches of internal / external carotid artery
(1) Direct shunt = direct communication between cavernous segment of ICA + cavernous sinus
Etiology:
(1) Trauma: laceration of ICA within cavernous sinus
(a) usually due to basal skull fracture (cavernous ICA + small cavernous branches fixed to dura)
(b) penetrating trauma
(c) surgery
(2) Spontaneous: rupture of an intracavernous ICA aneurysm (in atherosclerosis, Ehlers-Danlos syndrome, osteogenesis imperfecta, pseudoxanthoma elasticum)
(3) Dural sinus thrombosis
Age: any
• classic triad:
• pulsatile exophthalmos, conjunctival chemosis / edema
• persistent auscultatory orbital bruit
• restricted extraocular movement
• decrease in vision ← increase in intraocular pressure (50%) / cranial nerve deficits = indication for EMERGENT TREATMENT
(2) Indirect shunt = communication between dural branch of ICA / ECA + cavernous sinus
Age: 40–60 years; M < F
Etiology: atherosclerosis
• proptosis, loss of vision
√ ± visualization of feeding dural branches of ECA / ICA
Route of drainage:
(a) superior ophthalmic vein (common)
(b) contralateral cavernous sinus
(c) petrosal sinus
(d) cortical veins (rare)
√ dilatation + tortuosity of ipsilateral superior ophthalmic vein, facial veins, internal jugular vein
√ enlargement of dural venous sinuses ← increased venous flow + pressure
√ enlarged edematous extraocular muscles
√ focal asymmetric / diffuse enlargement of cavernous sinus
√ occasionally sellar erosion / enlargement
√ enlargement of superior orbital fissure (in chronic phase)
√ stretching of optic nerve
√ proptosis
√ subchoroidal effusion
US:
√ arterial flow in cavernous sinus + superior ophthalmic vein
√ early opacification of cavernous sinus
MR:
√ flow voids in cavernous sinus
Angio:
√ ipsilateral ICA contrast injection shows wall of ICA to be incomplete
√ contralateral ICA contrast injection + compression of involved ICA
√ early opacification of veins of cavernous sinus
√ retrograde flow through dilated superior ophthalmic vein
Rx: transvenous / transarterial coil ablation ± stent placement; latex / silicone balloon detached inside cavernous sinus to plug laceration (→ ocular signs resolve within 7–10 days with successful treatment)
DDx: cavernous sinus thrombosis, enhancing cavernous sinus mass (meningioma, metastasis)
Dural AV Fistula
Arterial supply: artery normally feeding meninges (meningeal artery) / bone / muscles
Draining vein: venules within wall of dural sinus / cortical vein
Cause: dural sinus thrombosis → collateral revascularization
Prevalence: 10–15% of intracranial AV shunts
Peak age: 20–40 years; M=F
• pulsatile tinnitus
Borden classification:
• benign fistula (Borden type 1) → no cortical venous reflux → no neurologic deficits
• malignant fistula (Borden type 2 & 3) → with cortical venous reflux
• intracranial hemorrhage, seizure, dementia
• focal neurologic symptoms due to venous congestion / rupture of venous pouches; altered consciousness
Location: cavernous sinus (20–40%), transverse / sigmoid sinus (20–60%), tentorium (12–14%), superior sagittal sinus (8%), anterior fossa (2–3%)
CECT:
√ multiple small vessels within wall of thrombosed / partially recanalized stenotic dural venous sinus
√ prominent feeding meningeal artery:
(a) ECA → dural / transosseous branch
(b) ICA / vertebral artery → tentorial / dural branch
√ enlarged draining veins
√ dilated transcalvarial channels ← transosseous feeding artery
MR:
√ dilated cortical veins (= pseudophlebitic pattern):
√ abnormal enhancing tubular structures
√ flow voids within cortical sulci
√NO true nidus within brain parenchyma
Rx: observation, embolization, surgical resection
DDx: dural venous sinus thrombosis with prominent collaterals, pial AV malformation, pial AV fistula
Pial AV Fistula
= often high-flow lesions with direct fistulous communication between a pial artery + a vein WITHOUT intervening nidus
Arterial supply: enlarged pial artery
Draining vein: enlarged draining vein / capillary bed
Prevalence: 5% of all brain AVMs
Associated with: hereditary hemorrhagic telangiectasia (frequent)
Location: brain surface
Cause: trauma, genetically dysregulated angiogenesis
√ dilated vessels of brain surface (from MCA, ACA, PCA)
√ asymmetric dilatation of pial feeder artery
√ dilated often (serpentine) varicose draining vein
√ ± dilated venous pouches outside brain parenchyma
√ NO nidus / classic intraparenchymal tangle of vessels
√ ± spontaneous intracranial hemorrhage
Rx: embolization of draining vein at fistula
DDx: AV malformation, dural AV fistula, vein of Galen malformation
ARTERIOVENOUS MALFORMATION
= congenital abnormality consisting of abnormal dilated closely packed pathologic vessels → shunting of blood from arterial to venous side without intermediary capillary bed
Risk of future hemorrhage:
(a) evidence of old hemorrhage (gradient-echo T2 sequence)
(b) angioarchitectural weak points:
› aneurysm: (1) intranidal (2) posterior fossa location
› venous caliber: (3) ectasia + (4) stenosis
› venous drainage: (5) deep + (6) single
◊ Imaging report should mention these risk factors!
Risk of nonhemorrhagic neurologic deficit:
high-flow shunt, venous congestion / outflow obstruction, long pial course of draining vein, perifocal / perinidal gliosis, mass effect / hydrocephalus, arterial steal
Classic Brain (Pial) AVM
◊ Most common type of symptomatic vascular malformation!
Diagnostic criteria:
(a) presence of a nidus = racemose tangle of abnormal dilated tortuous arteries + veins embedded within parenchyma
› glomerular / compact nidus = abnormal vessels without any interspersed normal brain tissue
› diffuse / proliferative nidus = interspersed normal brain parenchyma (2–4%)
(b) early venous drainage
Histo: affected arteries have thin walls (no elastica, small amount of muscularis)
Prevalence: 0.02–0.15% for sporadic AVM; 2% for syndromic AVM (hereditary hemorrhagic telangiectasia, cerebrofacial AV metameric syndrome)
Peak age: 20–40 years; 80% by end of 4th decade; 20% < 20 years of age; M=F
Associated with: aneurysm in feeding artery in 10%
• headaches, seizures (nonfocal in 40%), mental deterioration
• progressive hemispheric neurologic deficit (50%)
• ictus from acute intracranial hemorrhage (50%): multicompartmental in 31%, subarachnoid in 30%, parenchymal in 23%, intraventricular in 16%
Location: usually solitary; in 2% multiple
(a) supratentorial (90%): parietal > frontal > temporal lobe > paraventricular > intraventricular region > occipital lobe
(b) infratentorial (10%)
Site: (a) superficial / cortical:
› supply via pial arteries = branches of ACA, MCA, PCA;
› drainage via cortical veins
(b) deep / ventricular:
› supply via lenticulostriate, thalamoperforator branches, anterior / medial / lateral / posterior choroidal arteries
› drainage via deep venous system
(a) pial branches of ICA in 73% of supratentorial location, in 50% of posterior fossa location
(b) dural branches of ECA in 27% with infratentorial lesions
(c) mixed
√ NO mass effect (due to replacement of normal brain tissue) unless complicated by hemorrhage + edema:
√ intraparenchymal / intraventricular / subarachnoid hemorrhage
√ adjacent parenchymal atrophy ← vascular steal + ischemia
Skull film:
√ speckled / ringlike calcifications (15–30%)
√ thinning / thickening of skull at contact area with AVM
√ prominent vascular grooves on inner table of skull (= dilated feeding arteries + draining veins) in 27%
NECT:
√ irregular lesion with large feeding arteries + draining veins
√ mixed density (60%): dense large vessels + hemorrhage + calcifications
√ isodense lesion (15%): recognizable by mass effect
√ low density (15%): brain atrophy due to ischemia
√ not visualized (10%)
CECT:
√ tangle of intensely enhancing tubular structures = nidus ← tortuous dilated vessels (in 80%):
√ No avascular spaces within AVM
√ rapid shunting with veins seen during “arterial” phase
√ ± interspersed internal focal isoattenuating areas ← normal brain parenchyma (in diffuse subtype)
√ lack of mass effect / edema (unless thrombosed / bleeding)
√ No enhancement if thrombosed
√ thickened arachnoid covering
MR:
√ flow void ← rapid arteriovenous shunting (imaging with GRASS gradient echo + long TR sequences)
√ 3-D TOF demonstrates feeding arteries + nidus + draining veins
| Pitfalls: | (1) | signal void in tortuous vessels |
| (2) | nonvisualization of draining veins resulting from spin saturation | |
| (3) | difficulty differentiating blood flow from blood clot |
Angio:
√ grossly dilated efferent + afferent vessels with a racemose tangle (“bag of worms”)
√ arteriovenous shunting into at least one early draining vein
√ negative angiogram ← compression by hematoma / thrombosis
Cx: (1) Hemorrhage (common): bleeding on venous side ← increased pressure / ruptured aneurysm (5%)
(2) Infarction
Rx: embolization, stereotactic radiosurgery, microsurgery
Prognosis: 10% mortality; 30% morbidity
Risk of hemorrhage: lifelong; increasing yearly by 2–4%; increasing to 6% in year following 1st bleed + 25% in year following 2nd bleed
DDx: glioblastoma with AV shunting, dural AV fistula, cerebral proliferative angiopathy
Cerebral Proliferative Angiopathy
= diffuse nidus type AVM
Prevalence: 2–4%
Mean age: 20 years; M÷F = 1÷2
• progressive neurologic deficit
• transient ischemic attack, seizure, headaches
Histo: proliferative “nidus” composed of multiple arteries with intervening gliotic brain parenchyma between vessels
Pathophysiology: cortical ischemia → endothelial proliferation + angiogenesis
Location: often entire lobe / brain hemisphere
Vascular supply:
(a) arterial feeders of normal size / only moderately enlarged + associated stenoses
(b) extensive transdural supply through branches of ECA
(c) lack of clear early venous drainage
MR:
√ multiple flow voids
√ contrast-enhanced tubular structures
√ normal brain parenchyma interspersed between abnormal vessels
Angio:
√ relatively normal-sized arterial branches
√ lack of early venous drainage
√ extensive transdural supply via middle meningeal artery
Cerebrofacial Arteriovenous Metameric Syndrome
= CAMS = WYBURN-MASON SYNDROME = BONNET-DECHAUME-BLANC DISEASE
Cause: somatic mutation occurring in region of neural crest / adjacent cephalic mesoderm before migration of precursor cells to their final location = segmental neurovascular syndrome
Classification:
CAMS type 1: involves medial prosencephalon → AVMs in corpus callosum, hypothalamus (hypophysis), nose
CAMS type 2: involves lateral prosencephalon → AVMs in occipital lobe, optic tract including thalamus, retina, maxilla
CAMS type 3: involves rhombencephalon, → AVMs in cerebellum, pons, mandible
Clue: multiple AVMs in brain parenchyma + facial region in a segmental distribution
Age: childhood
• rarely manifest with hemorrhage
• symptoms related to facial AVMs:
• progressive vision loss resulting in blindness
• severe bleeding from teeth and gums
• cosmetic problems like facial asymmetry
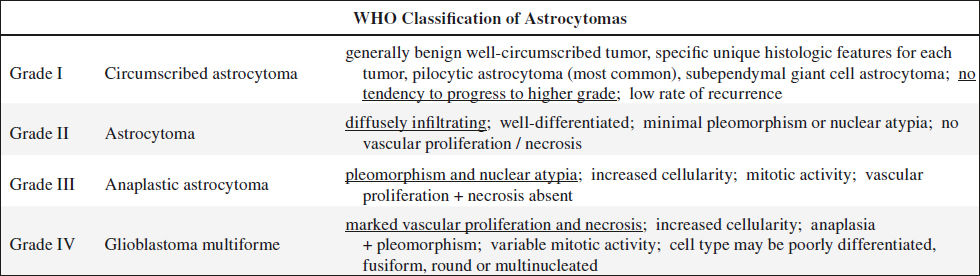
ASTROCYTOMA
Incidence: 70–75% of all primary intracranial tumors; most common brain tumor in children (40–50% of all primary pediatric intracranial neoplasms)
Distribution: proportional to amount of white matter
Location:
cerebral hemisphere (lobar), thalamus, pons, midbrain, may spread across corpus callosum; no particular lobar distribution
(a) in adults: central white matter of cerebrum (15–30% of all gliomas)
(b) in children: cerebellum (40%), brainstem (20%), supratentorial (30%)
Well-differentiated = Low-grade Astrocytoma
Incidence: 9% of all primary intracranial tumors; 10–15% of gliomas
Age: 20–40 years; M > F
Path: benign nonmetastasizing; poorly defined borders with infiltration of white matter + basal ganglia + cortex; NO significant tumor vascularity / necrosis / hemorrhage; blood-brain barrier may remain intact
Histo: homogeneous relatively uniform appearance with proliferation of well-differentiated multipolar fibrillary / protoplasmic astrocytes; mild nuclear pleomorphism + mild hypercellularity; rare mitoses
Location: posterior fossa in children, supratentorial in adults (typically lobar)
√ may develop a cyst with high-protein content (rare)
CT:
√ usually hypodense lesion with minimal mass effect + minimal / NO peritumoral edema
√ well-defined tumor margins
√ central calcifications (15–20%)
√ minimal / no contrast enhancement (normal capillary endothelial cells)
MR:
√ well-defined hypointense lesion with little mass effect / vasogenic edema / heterogeneity on T1WI
√ hyperintense on T2WI
√ little / no enhancement on Gd-DTPA
√ cyst with content hyperintense to CSF ← protein
√ hyperintense area within tumor mass ← paramagnetic effect of methemoglobin
√ inhomogeneous gadolinium-DTPA enhancement of tumor nodule
Angio:
√ majority avascular
Prognosis: 3–10 years postoperative survival; may convert into more malignant form several years later
Anaplastic Astrocytoma
Incidence: 11% of all primary intracranial neoplasms; 25% of gliomas
Path: frequently vasogenic edema; NO necrosis / hemorrhage
Histo: less well differentiated with greater degree of hypercellularity + pleomorphism, multipolar fibrillary / protoplasmic astrocytes; mitoses + vascular endothelial proliferation common
Location: typically frontal + temporal lobes
MR:
√ moderate mass effect
√ well-defined slightly heterogeneous hypointense lesion on T1WI with prevalent vasogenic edema
√ hyperintense on T2WI
√ ± enhancement on Gd-DTPA
Prognosis: postoperative survival of 2 years
Cerebellar Astrocytoma
= CEREBELLAR PILOCYTIC ASTROCYTOMA
2nd most frequent tumor of posterior fossa in children
Incidence: 10–20% of pediatric brain tumors
Histo: mostly grade I
Age: children > adults; no specific age peak; M÷F = 1÷1
Path:
(1) cystic lesion with tumor nodule (“mural nodule”) in cyst wall (50%); (midline astrocytomas cystic in 50%, hemispheric astrocytomas cystic in 80%)
(2) solid mass with cystic (= necrotic) center (40–45%)
(3) solid tumor without necrosis (< 10%)
• hydrocephalus, headache, vomiting, neck pain, 6th nerve palsy
• blurred vision, diplopia, papilledema, nystagmus
• cerebellar signs: truncal ataxia, dysdiadochokinesia appendicular dysmetria, gait disturbance
Location: originating in midline with extension into cerebellar hemisphere (29–53%), vermis (16–71%) > tonsils > brainstem (34%)
√ calcifications (20%): dense / faint / reticular / punctate / globular; mostly in solid variety
√ may develop extreme hydrocephalus (quite large when finally symptomatic)
CT:
√ round / oval cyst with density of cyst fluid > CSF
√ round / oval / plaquelike mural nodule with intense homogeneous enhancement
√ cyst wall slightly hyperdense + nonenhancing (= compressed cerebellar tissue)
√ uni- / multilocular cyst (= necrosis) with irregular enhancement of solid tumor portions
√ round / oval lobulated fairly well-defined iso- / hypodense solid tumor with hetero- / homogeneous enhancement
MR:
√ hypointense on T1WI + hyperintense on T2WI
√ enhancement of solid tumor portion
Angio:
√ avascular
Prognosis:
malignant transformation exceedingly rare
– 40% 25-year survival rate for solid cerebellar astrocytoma
– 90% 25-year survival rate for cystic juvenile pilocytic astrocytoma
DDx of solid astrocytoma:
(1) Medulloblastoma (hyperdense mass, noncalcified)
(2) Ependymoma (4th ventricle, 50% calcify)
DDx of cystic astrocytoma:
(1) Hemangioblastoma (lesion < 5 cm)
(2) Arachnoid cyst
(3) Trapped 4th ventricle
(4) Megacisterna magna
(5) Dandy-Walker cyst
Pilocytic Astrocytoma
= JUVENILE PILOCYTIC ASTROCYTOMA
= most benign histologic subtype of astrocytoma without progression to high-grade glioma
Incidence: 0.6–5.1% of all intracranial neoplasms
◊ Most common pediatric CNS glioma; 85% of all cerebellar + 10% of all cerebral astrocytomas in children
Age: predominantly in children + young adults; 75% in first 2 decades of life; peak age between birth and 9 years of age; M÷F = 1÷1
Histo: biphasic pattern of compact bipolar pilocytic (hairlike) astrocytes arranged mostly around vessels + loosely aggregated protoplasmic astrocytes undergoing microcystic degeneration
Associated with: neurofibromatosis type 1
Location: in / near midline
common: cerebellum, optic nerve / chiasm, hypothalamus (around 3rd ventricle)
less common: cerebral hemispheres (adults), cerebral ventricles, velum interpositum, spinal cord
Site: near ventricles (82%)
Imaging patterns:
(1) Cyst with intensely enhancing mural nodule (67%)
(a) nonenhancing cyst wall (21%)
(b) enhancing cyst wall (46%)
(2) Solid mass (33%)
(a) central nonenhancing necrotic zone (16%)
(b) minimal / no cystic component (17%)
CT:
√ well-demarcated smoothly marginated round / oval mass with cystic features
√ occasional calcifications
√ intense enhancement (94%)
√ multilobulated / dumbbell appearance along optic pathway
√ mural tumor nodule located in wall of cerebellar cyst
MR:
√ T1-isointense + T2-hyperintense to normal brain
√ small rim of vasogenic edema (low biologic activity)
√ increased heterogeneous signal intensity on early Gd-DTPA-enhanced T1WI; homogeneous enhancement on delayed images
Prognosis: relatively benign clinical course, almost never recurs after surgical excision; 94% (79%) postsurgical 10-year (20-year) survival; NO malignant transformation to anaplastic form
DDx: metastasis, hemangioblastoma, atypical medulloblastoma
Brainstem Pilocytic Astrocytoma
• nausea, vomiting, ataxia, torticollis
• papilledema, nystagmus, 6th & 7th nerve palsy
√ exophytic extension from dorsal surface of brainstem
√ obliteration of 4th ventricle
DDx: fibrillary astrocytoma (dismal prognosis)
Hypothalamic Pilocytic Astrocytoma
= HYPOTHALAMIC GLIOMA
• obesity, diabetes insipidus ← hypothalamic-pituitary dysfunction
• diencephalic syndrome (= emaciation despite normal / slightly decreased caloric intake, alert appearance, hyperkinesis, irritability, normal / accelerated growth)
• hemiparesis ← compression of corticospinal tracts
√ hydrocephalus
Prognosis: may regress spontaneously
Cerebral Pilocytic Astrocytoma
• headache, seizure activity, hemiparesis
• ataxia, nausea, vomiting
Location: temporal lobe
Optic Pathway Pilocytic Astrocytoma
= OPTIC NERVE GLIOMA
Location: optic nerve / chiasm
◊ Most common tumor in NF1 population (15–21%); NF1 diagnosed in ⅓ of all optic pathway gliomas; NF1 diagnosed in 40–70% of all tumors in this region; 1.5–3.5% of all orbital neoplasms; ²/³ of all neoplasms of the optic nerve
Age: < 6 years; M÷F = 2÷1
• visual loss / visual-field deficit, optic disk pallor, optic nerve atrophy ← axonal damage + ischemia
• precocious puberty (39%) in NF1 patients
Pleomorphic Xanthoastrocytoma
= superficially located supratentorial tumor that involves leptomeninges
Prevalence: 1% of all brain neoplasms
Age: average age of 26 years (range, 5–82 years)
Path: circumscribed tumor attached to meninges with infiltration into surrounding brain
Histo: pleomorphic spindled tumor cells (reactive to glial fibrillary acidic protein) with intracytoplasmic lipid (xanthomatous) deposits in a dense intercellular reticulin network; giant cells; eosinophilic granular bodies; WHO grade II tumor
• long history of seizures (71%)
Location: supratentorial (98%): temporal (49%) / parietal (17%) / frontal (10%) / occipital (7%) lobe; thalamus; cerebellum; spinal cord
◊ Its PERIPHERAL LOCATION is the single most consistent imaging feature
√ cystic (48%) supratentorial mass with mural nodule
√ intense enhancement of solid portions
√ CHARACTERISTIC involvement of leptomeninges (71%)
√ peritumoral vasogenic edema / calcification / skull erosion are uncommon
CT:
√ hypo- / isoattenuating mass
MR:
√ hypo- to isointense mass relative to gray matter on T1WI
√ hyper- to isointense mass on T2WI
Rx: surgical resection (unresponsive to chemotherapy + radiation therapy)
Prognosis: 81% (70%) 5-year (10-year) survival rate; high rate of recurrence; malignant transformation in 20%
DDx: meningioma, glioblastoma multiforme, oligodendroglioma, metastatic disease, infection
ATAXIA-TELANGIECTASIA
= autosomal recessive disorder characterized by telangiectasias of skin + eye, cerebellar ataxia, sinus + pulmonary infections, immunodeficiencies, propensity to develop malignancies
Incidence: 1÷40,000 livebirths
Path: neuronal degradation + atrophy of cerebellar cortex (? from vascular anomalies)
• cerebellar ataxia at beginning of walking age
• progressive neurologic deterioration
• oculomotor abnormalities, dysarthric speech, choreoathetosis, myoclonic jerks
• mucocutaneous telangiectasias: bulbar conjunctiva, ears, face, neck, palate, dorsum of hands, antecubital + popliteal fossa
• recurrent bacterial + viral sinopulmonary infections
√ cerebellar cortical atrophy: diminished cerebellar size, dilatation of 4th ventricle, increased cerebellar sulcal prominence
√ cerebral hemorrhage ← rupture of telangiectatic vessels
√ cerebral infarct ← emboli shunted through vascular malformations in lung
Cx: (1) Bronchiectasis + pulmonary failure (most common cause of death)
(2) Malignancies (10–15%): lymphoma, leukemia, epithelial malignancies
BENIGN MACROCEPHALY OF INFANCY
= BENIGN ENLARGEMENT OF SUBARACHNOID SPACES = BENIGN EXTRAAXIAL COLLECTIONS OF INFANCY = EXTERNAL HYDROCEPHALUS
Cause: defective reabsorption of CSF at arachnoid villi; commonly familial with autosomal dominant inheritance
Age: presentation between 3 and 12 months
• infant with macrocephaly (head circumference > 90th percentile)
• delayed motor development, hypotonia (in up to 30%)
Location: bilateral frontoparietal area + interhemispheric fissure + sylvian fissure + basal cisterns
√ enlarged subarachnoid spaces
√ “floating” cortical veins
√ NO / mild ventricular enlargement
Cx: subdural hematoma in response to minor impacts
Prognosis: self-limiting transient development that usually resolves by 2–3 years
DDx: (1) Cerebral atrophy (diffuse sulcal prominence not localized to frontoparietal area)
(2) Spontaneous subdural hematoma (12%)
BINSWANGER DISEASE
= ENCEPHALOPATHIA SUBCORTICALIS PROGRESSIVA = LEUKOARIAOSIS = SUBCORTICAL ARTERIOSCLEROTIC ENCEPHALOPATHY (SAE)
Cause: arteriosclerosis affecting the poorly collateralized distal penetrating arteries (perforating medullary arteries, thalamoperforators, lenticulostriates, pontine perforators); positive correlation with hypertension + aging
Path: ischemic demyelination / infarction
Age: > 60 years
• psychiatric changes, intellectual impairment, slowly progressive dementia, transient neurologic deficits
• seizures, spasticity, syncope
Location: periventricular white matter, centrum semiovale, basal ganglia; sparing of subcortical white matter “U” fibers + corpus callosum
√ multifocal hypodense lesions (periventricular, centrum semiovale) with sparing of U fibers
√ lacunar infarcts in basal ganglia
√ sulcal enlargement + dilated lateral ventricles (brain atrophy)
MR:
√ focal areas of increased signal intensity on T2WI (= “unidentified bright objects”)
DDx: leukodystrophy, progressive multifocal leukoencephalopathy, multiple sclerosis
BLAKE POUCH CYST
= embryonic midline outpouching of superior medullary velum extending inferior + posterior to vermis into cisterna magna
Blake pouch = rudimentary 4th ventricular tela choroidea membrane as a normal transient structure that regresses by 12 weeks GA to form the foramen of Magendie (communication between 4th ventricle + subarachnoid space)
Cause: failure of fenestration of foramen of Magendie
Age: neonate
√ retrocerebellar / infraretrocerebellar cyst = diverticulum of enlarged 4th ventricle
√ enhancing structure along anterosuperior aspect of cyst inferior to vermis = displaced choroid plexus (on SAG contrast-enhanced T1WI)
√ mild indentation of inferior vermis / caudomedial aspects of cerebellar hemispheres ← mass effect of cyst
√ tetraventricular hydrocephalus
Pertinent negatives:
√ NO supratentorial morphologic abnormalities aside from hydrocephalus
√ posterior fossa + cerebellum of normal size + shape
Prognosis: favorable (shunt-related complications possible)
DDx: megacisterna magna
BRAIN / CEREBRAL DEATH
Confirmatory tests of absent blood flow function:
1. Four-vessel contrast angiography (carotid and vertebral aa.)
2. Radionuclide cerebral blood flow angiography
3. CECT
4. Ultrasonic echoencephalography
5. Doppler ultrasound
6. Digital subtraction angiography (DSA)
7. MRI
◊ Administration of contrast may damage brain / kidney OR compromise tissue function
DDx by EEG:
Severe barbiturate intoxication (may produce a flat EEG response in the absence of brain death)
Radionuclide Angiography
= 1st-line test for cerebral perfusion; can be performed at the ICU bed site
Indication:
(1) Prior to organ harvest
(2) Hypothermia / drug intoxication interfering with clinical + EEG assessment of brain activity
(3) Brain death as a possible result of criminal activity
Pathophysiology: increased intracranial pressure above systemic arterial pressure results in markedly decreased cerebral perfusion → thrombosis → total cerebral infarction
Path: severe brain edema, diffuse liquefactive necrosis
Agent:
› nondiffusable hydrophilic 99mTc pentetic acid (99mTc-DTPA)
◊ Absence of effective cerebral perfusion at planar scintigraphy does NOT equate with brain death as blood flow to brainstem cannot be adequately assessed with 99mTc pentetic acid!
› diffusible brain imaging agents that cross the normal blood brain barrier
» lipophilic 99mTc bicisate (99mTc-ECD)
» lipophilic 99mTc exametazime (99mTc-HMPAO)
√ activity stops abruptly at the skull base = lack of activity in distribution of anterior and middle cerebral arteries + superior sagittal sinus (SSS) during 1st pass (= angiographic phase):
√ NO / faint activity in SSS on static image with 99mTc pentetic acid
√ absence of activity in cerebrum on delayed images with 99mTc bicisate / 99mTc exametazime
√ sagittal sinus not visualized
N.B.:
√ common carotid arteries must be clearly visualized on 1st pass which confirms a good technically adequate bolus
√ activity in face (“hot nose” sign) / scalp must not be mistaken as focal brain activity
False negative:
decrease in intracranial pressure may allow continuous flow through intracranial arteries and has been observed in
(a) extensive liquefactive brain necrosis
(b) incompletely ossified skull in child
(c) open head injury in adults
CANAVAN DISEASE
= SPONGIFORM LEUKODYSTROPHY
= rare form of leukodystrophy as an autosomal recessive disorder, most common in Ashkenazi Jews
Incidence: < 100 reported cases
Cause: deficiency of aspartoacyclase leading to accumulation of N-acetylaspartic acid in brain, plasma, urine, CSF
Histo: spongy degeneration of white matter with astrocytic swelling + mitochondrial elongation
Age: 3–6 months
• marked hypotonia, spasticity, seizures
• progressive megalencephaly
• failure to attain motor milestones, intellectual failure
• optic atrophy with blindness, swallowing impairment
√ diffuse symmetric white matter abnormality
√ may involve basal ganglia
√ cortical atrophy
CT:
√ low-density white matter
MR:
√ white matter hypointense on T1WI + hyperintense on T2WI
Prognosis: death in 2nd–5th year of life
Dx: (1) elevation of N-acetylaspartic acid in urine
(2) deficiency of aspartoacyclase in cultured skin fibroblasts
CAPILLARY TELANGIECTASIA
= CAPILLARY ANGIOMA
= nest of dilated capillaries separated by normal neural tissue; commonly “cryptic”
May be associated with:
hereditary Rendu-Osler-Weber syndrome, ataxia-telangiectasia syndrome, irradiation (latency period of 5 months to 22 years)
Age: typically in elderly
• usually asymptomatic (incidental finding at necropsy)
Location: mostly in pons / midbrain > cerebral cortex > spinal cord; usually multiple / may be solitary
√ poorly defined area of dilated vessels (resembling petechiae)
√ best delineated with MR (due to hemorrhage) with focus of increased signal intensity on contrast-enhanced studies
Cx: punctate hemorrhage (uncommon); gliosis + calcifications (rare)
Prognosis: bleeding in pons (usually fatal)
DDx: cavernous angioma (identical image signature)
CAVUM VELI INTERPOSITI CYST
= cyst of ventricular roof in between two-layered tela choroidea
√ distortion of posterior superior contour of 3rd ventricle mimicking an obstructed 3rd ventricle
√ cyst of triangular contour on axial images
√ superior displacement of fornix
√ inferolateral displacement of internal cerebral veins
CENTRAL NEUROCYTOMA
= name reserved for neurocytoma that occurs in ventricles
Incidence: 0.25–0.5% of intracranial tumors
Origin: ? bipotential progenitor cells capable of both neuronal + glial differentiation
Histo: solid sheets / large lobules of small round to ovoid neoplastic cells with delicate vascular network + intervening irregular patches of fibrillary neuropils; pineocytomatous rosettes (not in oligodendroglioma)
Immunohisto: synaptophysin, neuron-specific enolase
Mean age: 29 years (range, 8 days to 67 years); M=F
• symptoms of increased intracranial pressure
Location: (a) lateral ventricle ± extension into 3rd ventricle
Site: septum pellucidum, ventricular wall
(b) extraventricular: parenchyma, cerebellum, spinal cord
√ well-circumscribed lobulated mass
√ frequently “bubbly” appearance ← presence of multiple cysts
√ calcifications (50%)
√ moderate to strong enhancement
CT:
√ hyperattenuating lesion
MR:
√ lesion T1-isointense + T2-hyperintense to gray matter
√ ± prominent flow voids
√ increased T2 signal intensity in adjacent periventricular white matter
MR spectroscopy:
√ presence of glycine (3.55 ppm)
Rx: usually curative resection
Cx: recurrence after resection, CSF dissemination
CEPHALOCELE
= mesodermal defect of calvarial suture + dura with extracranial extension (= herniation) of intracranial structures and persistent connection to subarachnoid space
| Cranial meningocele: | = | herniation of meninges + CSF only |
| Encephalocele | = | herniation of meninges |
(Meningoencephalocele) + CSF + neural tissue
Nomenclature: based on origin of their roof + floor
eg, frontonasal: frontal bone = roof, nasal bone = floor
Prevalence:
1–4÷10,000 live births; 5–6–20% of all craniospinal malformations; predominant neural axis anomaly in fetuses spontaneously aborted < 20 weeks GA; 3% of fetal anomalies detected with MS-AFP screening; 6% of all detected neural tube defects in fetuses
Cause:
failure of surface ectoderm to separate from neuroectoderm early in embryonic development (3rd week GA)
@ Skull base
(1) faulty closure of neural tube (without mesenchyme membranous cranial bone cannot develop)
(2) failure of basilar ossification centers to unite
@ Calvarium
(1) defective induction of bone
(2) pressure erosion of bone by intracranial mass / cyst
In 60% associated with:
1. Spina bifida (7–30%)
2. Corpus callosum dys- / agenesis
3. Chiari malformation
4. Dandy-Walker malformation
5. Cerebellar hypoplasia
6. Amniotic band syndrome: multiple irregular asymmetric off-midline encephaloceles
7. Migrational abnormalities
8. Chromosomal anomalies in 44% (trisomy 18)
• MS-AFP elevated in 3% (skin-covered in 60%)
• CSF rhinorrhea; meningitis
Prognosis: dependent on associated malformations + size and content of lesion; 21% liveborn; 50% survival of liveborns, 74% retarded
◊ The larger the brain volume the poorer the outcome
Risk of recurrence: 3% (25% with Meckel syndrome)
DDx: teratoma, cystic hygroma, iniencephaly, scalp edema, hemangioma, branchial cleft cyst, cloverleaf skull
Occipital Encephalocele (75%)
Most common encephalocele in Western Hemisphere
Associated with:
(1) Meckel-Gruber syndrome
= occipital encephalocele + microcephaly + cystic dysplastic kidneys + polydactyly
(2) Dandy-Walker malformation
(3) Chiari malformation
(4) Callosal + migrational anomalies
• external occipital mass
Location: supra- and infratentorial structures involved with equal frequency
√ skull defect (visualized in 80%)
√ flattening of basiocciput
√ ventriculomegaly
√ “lemon” sign = inward depression of frontal bones (33%)
√ cyst-within-a-cyst (ventriculocele = herniation of 4th ventricle into cephalocele)
√ acute angle between mass + skin line of neck and occiput
DDx: cystic hygroma
Sincipital Encephalocele (13–15%)
= FRONTOETHMOIDAL ENCEPHALOCELE
Most common variety in Southeast Asian population
Location: midface about dorsum of nose, orbits, and forehead
Cause: failure of anterior neuropore located near optic recess to close normally at 4th week GA
Types:
1. Nasofrontal (40–60%)
= herniation of dura mater through foramen cecum + fonticulus frontalis
Site: along nasal bridge between nasofrontal sutures into glabella
2. Nasoethmoidal (30%)
= persistent herniation of dural diverticulum through foramen cecum into prenasal space
Site: between nasal bone + nasal cartilage (beneath nasal bone + above nasal septum)
3. Naso-orbital
Site: between maxilla + lacrimal bone (= along medial orbit at level of frontal process of maxilla and ethmoid-lacrimal bone junction)
Common root: foramen cecum (= small ostium anterior to crista galli formed by closure of frontal + ethmoid bones)
Associated with: midline craniofacial dysraphism (dysgenesis of corpus callosum, interhemispheric lipoma, anomalies of neural migration, facial cleft, schizencephaly)
• obvious nonprogressive pulsatile mass
• broad nasal root, hypertelorism, nasal stuffiness, rhinorrhea
• change in size during crying / Valsalva maneuver
• positive Fürstenberg test = change in size during jugular compression
√ soft-tissue mass extending to glabella / nasal cavity
√ pedunculated intranasal mass extending from superomedial nasal cavity downward
√ enlarged foramen cecum
OB-US:
√ widened interorbital distance
CT:
√ bifid / absent crista galli
√ absent cribriform plate / frontal bone
MR:
√ isointense relative to gray matter
√ may be hyperintense on T2WI (due to gliosis)
N.B.: biopsy is CONTRAINDICATED (→ potential for CSF leaks, seizures, meningitis)
Risk of recurrence: 6% of congenital CNS abnormalities for younger siblings
Rx: complete surgical resection with repair of dura mater (NO neurologic deficit due to abnormal function of herniated brain)
DDx: (1) Dacryocystocele / nasolacrimal mucocele
(2) Nasal glioma (no subarachnoid connection on cisternography)
Sphenoidal Encephalocele (10%)
= BASAL ENCEPHALOCELE
Age: present at end of 1st decade of life
• clinically occult ← internal protrusion
• mass in nasal cavity, nasopharynx, mouth, posterior portion of orbit increasing with Valsalva
• mouth breathing due to nasopharyngeal obstruction
• diminished visual acuity with hypoplasia of optic disks
• hypothalamic-pituitary dysfunction
Associated with: agenesis of corpus callosum (80%)
Types:
(a) transethmoidal = through midline cribriform plate
(b) sphenoethmoidal = through sphenoid + ethmoid
(c) trans-sphenoidal = through floor of sella may be associated with: cleft palate
√ displacement of cavernous sinus (laterally), pituitary gland, hypothalamus, optic nerves, chiasm
(d) frontosphenoidal
(e) sphenopharyngeal = through sphenoid body
(f) sphenoorbital = through superior orbital fissure
(g) sphenomaxillary = through maxillary sinus
Parietal Encephalocele (10–12%)
Associated with: dysgenesis of corpus callosum, large interhemispheric cyst
√ hole in sphenoid bone (seen on submentovertex film)
√ cranium bifidum = cranioschisis = “split cranium” (= skull defect) = smooth opening with well-defined sclerotic rim of cortical bone
√ hydrocephalus in 15–80% (from associated aqueductal stenosis, Arnold-Chiari malformation, Dandy-Walker cyst)
√ nonenhancing expansile homogeneous paracranial mass
√ mantle of cerebral tissue often difficult to image in encephalocele (except with MR)
√ intracranial communication often not visualized
√ metrizamide / radionuclide ventriculography DIAGNOSTIC
√ microcephaly (20%)
√ polyhydramnios
DDx: (1) sonographic refraction artifact at skull edge
(2) clover leaf skull (± temporal bone partially absent)
CEREBRAL AMYLOID ANGIOPATHY
= deposition of β-amyloid protein in media + adventitia of small + medium-sized vessels of cerebral cortex, subcortex and leptomeninges
Age: increasing with age: 33% in 60–70 years, 75% in > 90 years
Path: fibrinoid necrosis, focal vessel wall fragmentation, microaneurysm → vessel leakage + frank hemorrhage; luminal narrowing → ischemic change
Histo: yellow-green birefringent (under polarized light) deposits along vessel wall with Congo red stain
Types: sporadic form (common), hereditary form (rare)
• asymptomatic (in many): underrecognized with petechial microhemorrhages ≤ 5 mm
• headaches, emesis, focal neurologic deficit, seizure, coma with macrohemorrhage > 5 mm
• transient ischemic attack, dementia
• normotensive elderly without trauma
√ acute / chronic intracerebral hemorrhage (ICH)
◊ Cerebral amyloid angiopathy represents 2% of all ICH
Location: cortical / subcortical in any lobe; sparing of deep white matter + basal ganglia + brainstem
√ may be associated with subarachnoid / subdural hemorrhage
√ leukoencephalopathy ± involvement of U-fibers
√ cerebral atrophy
MR:
√ multiple foci of marked signal loss at GRE imaging (most sensitive sequence for hemosiderin)
DDx: hypertensive hemorrhage (basal ganglia, thalami, brainstem)
CEREBRAL CAVERNOUS MALFORMATION
= CAVERNOUS ANGIOMA OF BRAIN = CAVERNOUS HEMANGIOMA = CAVERNOMA
= benign vascular hamartoma of immature blood vessels + intralesional hemorrhage
Cause: sporadic + solitary (⅔); hereditary + multiple (⅓)
Prevalence: 0.2–0.4% of general population
Associated with: ? developmental venous anomaly
Path: well-circumscribed nodule of honeycomblike dilated endothelial lined spaces separated by fibrous collagenous bands WITHOUT intervening neural tissue
Age: any; 3rd–6th decade (most common); M = F
• asymptomatic (most)
◊ Most common asymptomatic vascular malformation!
• headache, seizures (commonly presenting symptom), neurologic deficit (15%)
Location: cerebrum (mainly superficial subcortical in close contact with subarachnoid space / ventricles) > pons > cerebellum; solitary > multiple
√ NO obvious mass effect / edema
√ usually contain blood degradation products of different stages
√ slow blood flow in vascular channels
NECT:
√ small round / lobulated hyperdense region (CLUE)
√ minimal surrounding edema
√ extensive calcifications = hemangioma calcificans (20%)
CECT:
√ none / minimal / intense enhancement
√ low-attenuation areas due to thrombosed portions
MR (DIAGNOSTIC):
√ typically popcorn appearance with bright lobulated center on T1WI + T2WI
√ well-defined area of mixed signal intensity centrally (= “mulberry”-shaped lesion) with a mixture of:
√ increased signal intensity (= extracellular methemoglobin / slow blood flow / thrombosis)
√ decreased intensity (= deoxyhemoglobin / intracellular methemoglobin / hemosiderin / calcification)
√ surrounded by hypointense rim (= hemosiderin) on T2WI
Angio:
√ negative = “cryptic / occult vascular malformation”
Cx: hemorrhage of varying age
Risk of hemorrhage: 0.4–3.1% (4.3–6.5%) per year for sporadic (familial) cases
Rx: none; microsurgery (if symptomatic)
DDx: (1) Hemorrhagic neoplasm (edema, mass effect)
(2) Hypertensive hemorrhage
(3) Small AVM (thrombosed / small feeding vessels, associated hemorrhage)
(4) Capillary telangiectasia / angioma (no difference)
CEREBRAL VENOUS THROMBOSIS
= DURAL SINUS THROMBOSIS = VENOUS SINUS / SUPERIOR SAGITTAL SINUS THROMBOSIS
◊ The radiologist may be the first to suggest the diagnosis!
Annual Incidence: 2–7÷1,000,000
Cause: > 100 causes suggested
A. IDIOPATHIC = spontaneous (10–30%)
B. LOCAL CAUSE (= intrinsic / mechanical conditions of veins / dural sinuses)
› Septic causes (esp. in childhood): sinusitis, otitis, mastoiditis, sub- / epidural empyema, meningitis, encephalitis, brain abscess, face + scalp cellulitis
› Aseptic causes:
(a) Tumor compressing sinus: meningioma
(b) Trauma: fracture through sinus wall, brain damage, cranial surgery, jugular vein catheterization
C. SYSTEMIC CAUSE (= conditions that promote thrombosis)
› Septic causes: septicemia
› Aseptic causes:
(a) Low-flow state: CHF, CHD, dehydration, shock, surgery, immobilization
(b) Hypercoagulability: antithrombin III deficiency, antiphospholipid syndrome, protein S + C deficiency, pregnancy, peripartum state, oral contraceptives, malignancy, polycythemia vera, idiopathic thrombocytosis, thrombocytopenia, sickle cell disease, cryofibrinogenemia, disseminated intravascular coagulopathy
(c) Chemotherapy: eg, ARA-C, L-asparaginase
› Unusual causes: Behçet disease, AIDS, ulcerative colitis, SLE, nephrotic syndrome, sarcoidosis
Pathophysiology:
dural sinus thrombosis → thrombus propagation into cortical veins → venous congestion → cerebral venous infarction (in 50%) → vasogenic / cytotoxic edema → intracranial hemorrhage; occasionally hydrocephalus (→ decreased CSF absorption ← impaired function of arachnoid granulations)
Onset: acute = < 2 days (in 30%), subacute = 2–30 days (in 50%), chronic = > 30 days (20%)
• symptoms of intracranial hypertension (20–40%): headaches (75–95%), nausea, vomiting, visual blurring, papilledema
often confused with: tension headaches, migraine
• drowsiness, confusion, coma, decreased mentation, lethargy, obtundation, seizures, fever
• focal neurologic deficits = stroke symptomatology (dysphasia, cranial nerve palsy, cerebellar incoordination) ← frequently parenchymal changes
Location: superior sagittal sinus (62%) > L transverse sinus (45%) > R transverse sinus (41%) > sigmoid sinus (15%) > straight sinus (18%) > cortical veins (17%) > deep venous system (11%) > jugular bulb (8%) > vein of Galen (7%) > cavernous sinus (1%) > cerebellar veins (0.3%)
√ bilateral parasagittal hemispheric lesions ← superior sagittal sinus thrombosis
√ ipsilateral temporo-occipital + cerebellar lobe lesions ← transverse sinus thrombosis
√ bilateral thalamic lesions ← deep cerebral venous thrombosis
NECT (usually subtle findings):
√ hyperattenuating intravascular material (← acute blood clot) in sagittal sinus = “dense triangle” sign / straight sinus / cerebral cortical vein = “cord” sign lasting for 1–2 weeks (seen in only 20% ← variability in degree of thrombus attenuation)
DDx to hyperattenuated thrombus:
dehydrated patient, elevated hematocrit level, polycythemia, nonmyelinated brain in neonates, subjacent subarachnoid / subdural hemorrhage
√ subdural collection
√ stroke (often hemorrhagic)
◊ Thrombosis of intracranial dural sinuses, cortical / deep cerebral veins, cavernous sinus is an easily recognizable condition that accounts for 1% of acute cerebral infarcts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree