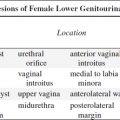
bilateral renal agenesis / dysplasia / infantile polycystic kidney disease, congenital nephrotic syndrome, congenital nephritis, perinatal hypoxia
Frequency: ATN + prerenal disease account for 75% of acute renal failure; 5–7% of all hospitalized patients
• asymptomatic
• elevated creatinine normal range: 0.5–1.0 mg/dL (about 45–90 μmol/L) for women + 0.7–1.2 mg/dL (60–110 μmol/L) for men
Prognosis: 20–70% mortality
Chronic Renal Failure (CRF)
= decrease in renal function over months / years
Incidence: end-stage renal disease in 0.01% of USA population; annually 85,000 patients undergo hemodialysis + 8,000 renal transplantations
Etiology:
A. INFLAMMATION / INFECTION
1. Glomerulonephritis
2. Chronic pyelonephritis
3. Tuberculosis
4. Sarcoidosis
B. VASCULAR
1. Renal vascular disease
2. Bilateral renal vein thrombosis
C. DYSPROTEINEMIA
1. Myeloma
2. Amyloid
3. Cryoglobulinemia
4. Waldenström macroglobulinemia
D. METABOLIC
1. Diabetes
2. Gout
3. Hypercalcemia
4. Hyperoxaluria
5. Cystinosis
6. Fabry disease
7. Calcinosis
E. CONGENITAL
1. Polycystic kidney disease
2. Multicystic dysplastic kidney
3. Medullary cystic disease
4. Alport syndrome
5. Infantile nephrotic syndrome
F. MISCELLANEOUS
1. Hepatorenal syndrome
2. Radiation
Musculoskeletal Manifestations of CRF
1. Renal osteodystrophy = combination of 2° HPT, osteoporosis, osteosclerosis, osteomalacia, soft-tissue and vascular calcifications
2. Aluminum toxicity (1–30%)
3. Amyloid deposition
Path: amyloid consists of β2-microglobulin
Organs: bone, tenosynovium (carpal tunnel syndrome), vertebral disk, articular cartilage + capsule, ligament, muscle
4. Destructive spondyloarthropathy (15%)
√ diskovertebral junction erosion + sclerosis
√ vertebral body compression
√ disk space narrowing
√ Schmorl node formation
√ lack of osteophytosis
√ facet involvement with subluxation
5. Tendon rupture
6. Crystal deposition disease
Type: calcium hydroxyapatite, CPPD, calcium oxalate, monosodium urate
7. Osteomyelitis + septic arthritis
8. Avascular necrosis (in up to 40%)
DIABETES INSIPIDUS
= characterized by daily production of very large volume of dilute urine (specific gravity < 1.005, < 200 mOsm/L)
Pituitary Diabetes Insipidus
= HYPOTHALAMIC DIABETES INSIPIDUS
= VASOPRESSIN-SENSITIVE DIABETES INSIPIDUS
= vasopressin (ADH) production is reduced to < 10%
Cause:
A. IDIOPATHIC (27%)
septooptic dysplasia / rare familial (autosomal dominant X-linked) / sporadic disorder
Histo: atrophic supraoptic nucleus
• never associated with anterior pituitary dysfunction
B. PITUITARY DESTRUCTION BY TUMOR / INFILTRATIVE DISORDER (32%):
in childhood: hypothalamic glioma, tuber cinereum hamartoma, craniopharyngioma, Langerhans histiocytosis, germinoma, leukemia, complication of meningitis
in adulthood: sarcoidosis, TB, metastasis
• in 60% associated with anterior pituitary dysfunction
C. PITUITARY DESTRUCTION BY SURGERY (20%)
• always associated with anterior pituitary dysfunction
D. HEAD INJURY (17%)
• in 20% associated with anterior pituitary dysfunction
◊ A lesion in the posterior pituitary will NOT produce diabetes insipidus, because it is simply the storage space for vasopressin
Psychogenic Water Intoxication
= compulsive intake of large amounts of fluid → inhibits normal vasopressin production
• water deprivation test
Nephrogenic Diabetes Insipidus
= poor reabsorption of water in collecting ducts ← end-organ resistance to vasopressin
Cause:
A. CONGENITAL
1. Rare X-linked recessive genetic disorder with unresponsiveness of tubules + collecting system to vasopressin (in infants + young males) with variable expression
2. Autosomal dominant form (rare)
B. ACQUIRED = nephrogenic DI syndrome
= disorders affecting the medulla / distal nephrons:
medullary + polycystic disease, sickle cell nephropathy, postobstructive uropathy, reflux nephropathy, chronic uremic nephropathy, unilateral renal artery stenosis, acute tubular necrosis, drug toxicity, analgesic nephropathy, hypokalemic + hypercalcemic nephropathy, amyloidosis, sarcoidosis
• symptoms in infancy:
• vomiting ← hypernatremic dehydration
• mental retardation
• caloric growth failure ← water favored over formula
• symptoms after infancy:
• increased fluid intake; avoiding urination
√ bilateral hydroureteronephrosis
Rx: thiazide diuretics, low-salt diet, encouragement of frequent micturition, indomethacin
PRIMARY ALDOSTERONISM
= inappropriate autonomous hypersecretion of aldosterone WITHOUT activation of renin-angiotensin-aldosterone axis
Frequency: 5–15% of unselected hypertensive patients
◊ Most common cause of secondary hypertension!
Biochemistry: mineralocorticoid production in zona glomerulosa
Cause: (main role of radiology)
(1) Aldosterone-producing adenoma (APA) 33–66%)
Age: often found in patients < 40 years
Size: < 2 cm (usually)
Rx: adrenalectomy
(2) Bilateral adrenal hyperplasia (BAH) 33–66%
Age: often found in older patients > 40 years
√ enlargement of both adrenal glands
Rx: aldosterone antagonists (eplerenone)
(3) Unilateral adrenal hyperplasia < 1%
Rx: adrenalectomy
(4) Adrenal carcinoma rare
(5) Type 1 familial hyperaldosteronism rare
(5) Type 2 familial hyperaldosteronism rare
• hypertension
• hyperaldosteronism, suppressed renin, hypernatremia
• abnormal aldosterone (↑)-renin (↓)ratio, metabolic alkalosis
• failure to suppress plasma aldosterone levels by oral salt-loading / IV administration of saline solution / fluorocortisone
CT (40–100% sensitive) & MR (70–100% sensitive):
√ small adrenal nodule:
√ macronodule > 10 mm
√ micronodule < 10 mm
√ mean adrenal limb width ≥ 5 mm = BAH
Adrenal vein sampling:
= blood samples taken from IVC + both adrenal veins during infusion of adrenocorticotropic hormone
√ cortisol ratio of adrenal vein÷IVC > 2÷1
√ lateralization ratio = adrenal vein÷adrenal vein:
√ > 4 = aldosterone hypersecretion
√ < 3 = BAH
NUC (131Iodine–6-β-iodomethylnorcholesterol = NP-59 or Selenium-75–6-β-selenomethylcholesterol):
› dexamethasone depression increases sensitivity
√ unilateral early uptake < 5 days = APA
√ symmetric early uptake < 5 days = BAH
√ symmetric late uptake > 5 days = normal
HYPERCALCEMIA
mnemonic: SHAMPOO DIRT
Sarcoidosis
Hyperparathyroidism, Hyperthyroidism
Alkali-milk syndrome
Metastases, Myeloma
Paget disease
Osteogenesis imperfecta
Osteopetrosis
D vitamin intoxication
Immobility
Renal tubular acidosis
Thiazides
POLYCYTHEMIA
Cause: increased level of erythropoietin (acting on erythroid stem cells) ← decrease in pO2; erythropoietin precursor is produced in juxtaglomerular epithelioid cells of kidney + converted in blood
A. RENAL
(a) intrarenal
1. Vascular impairment
2. Renal cell carcinoma (5%)
3. Wilms tumor
4. Benign fibroma
6. Polycystic kidney disease
(b) postrenal
1. Obstructive uropathy (14%)
B. EXTRARENAL
(a) liver disease
1. Hepatoma
2. Regenerating hepatic cells
(b) adrenal disease
1. Pheochromocytoma
2. Aldosteronoma
3. Cushing disease
C. CNS DISEASE
1. Cerebellar hemangioblastoma
D. LARGE UTERINE MYOMAS
Pertinent negatives: NOT in renal vein thrombosis, multicystic dysplastic kidney, medullary sponge kidney
ARTERIAL HYPERTENSION
A. PRIMARY / ESSENTIAL HYPERTENSION (85–90%)
B. SECONDARY HYPERTENSION
(a) Renal parenchymal disease (5–10%)
(b) Potentially curable secondary hypertension (1–2%)
› vascular
1. Renovascular disease 0.18–4.4%
2. Coarctation 0.6%
› hormonal
1. Pheochromocytoma 0.04–0.2%
2. Cushing syndrome 0.3%
3. Primary aldosteronism 0.01–0.4%
4. Hyperthyroidism
5. Myxedema
› renal
1. Unilateral renal disease
Renovascular Hypertension
= normalization of blood pressure following nephrectomy / reestablishment of normal renal blood flow (Diagnosis made in retrospect)
Prevalence: 1–5% of general population; 2nd most common cause of potentially curable hypertension
Pathophysiology:
usually > 50% stenosis at any level in renovascular bed → mildly reduced pressure in glomerular afferent arteriole (pressure falls precipitously in > 80% stenosis); reduced pressure → stimulates release of renin → followed by angiotensin-II and aldosterone causing
(a) constriction of efferent glomerular arterioles
(b) increase in systemic hypertension
(c) sodium retention
Cause:
1. Atherosclerosis (60–90%) in individuals > 50 years
2. Fibromuscular dysplasia (10–35%) in women < 40 years
3. Neurofibromatosis
4. Pheochromocytoma
5. Fibrous bands: congenital stenosis, retroperitoneal fibrosis, postradiation artery stenosis
6. Arteritis: Buerger disease, polyarteritis nodosa, Takayasu disease, thrombangiitis obliterans, syphilitic arteritis
7. Arteriovenous malformation / fistula
• renin-mediated hypertension ← renal ischemia distal to fistula
8. Thromboembolic disease: eg, atrial fibrillation, prosthetic valve thrombi, cardiac myxoma, paradoxical emboli, atheromatous emboli
9. Renal artery aneurysm
10. Extrinsic compression: eg, renal cyst, neoplasm, chronic subcapsular hematoma (= Page kidney)
11. Middle aortic syndrome, aortic dissection, dissecting aortic aneurysm
12. Posttraumatic renovascular hypertension
(a) occlusion of main renal artery
(b) significant stenosis by intimal flap
(c) severe renal contusion
(d) segmental renal artery branch injury
◊ Renal artery stenosis in 77% of hypertensive patients!
◊ Renal artery stenosis in 32–49% of normotensive patients!
◊ After restoration of normal renal blood flow 15–20% of patients remain hypertensive!
Clinical findings that suggest renovascular disease:
1. Onset of HTN < 30 years and > 50 years of age
2. Hypertension refractory to therapy
3. Accelerated / malignant hypertension
4. Unexplained large increases in blood pressure above previously controlled / baseline values
5. Symptomatic hypertension
| Rx: | (1) Relieving renal artery stenosis |
(2) Angiotensin-converting enzyme inhibitor |
Hypertension in Children
Prevalence: 1–3%
1. Coarse renal cortex scarring 36%
2. Glomerulonephritis 23%
3. Coarctation of aorta 10%
4. Renovascular disease 10%
5. Polycystic renal disease 6%
6. Hemolytic-uremic syndrome 4%
7. Catecholamine excess: pheochromocytoma, neuroblastoma 3%
8. Renal tumor 2%
9. Essential hypertension 3%
Renin Elevation
1. Juxtaglomerular cell tumor
2. Wilms tumor
3. Hypernephroma
4. Lung cancer
5. Paraovarian tumor
6. Fallopian tube adenocarcinoma
7. Epithelial liver hamartoma
8. Orbital hemangiopericytoma
9. Pancreatic cancer
10. Angiolymphoid hyperplasia
ARTERIAL HYPOTENSION
Cause: intrarenal hypovolemia, primary vasoconstriction, reduced glomerular filtration, depletion of intratubular urine volume
◊ May occur as a contrast reaction!
◊ Urogram reverts to normal after reversion of hypotension!
√ bilateral small smooth kidneys (compared with size on preliminary films)
√ increasingly dense nephrogram
√ usually NO opacification of collecting system
√ initially opacification of collecting system if hypotension occurs during contrast injection
URINARY TRACT INFECTION
= pure growths of > 100,000 organisms/mL urine
Prevalence: 3% of girls + 1% of boys during first 10 years of life
Underlying radiologic abnormality:
1. Vesicoureteral reflux = VUR (30–40%)
2. Obstructive uropathy (8%)
3. Reflux nephropathy / scar formation (6%)
◊ The prevalence of an underlying radiologic abnormality depends on age, sex, and frequency of previous infections!
Imaging objective:
1. Identify patients at risk for reflux nephropathy
2. Detect reflux nephropathy / scars
3. Detect obstructive uropathy
4. Minimize radiation, morbidity, and cost
VCUG:
for children < 5 years of age with infection; normal results in 60–70%
Renal cortical scintigraphy (DMSA / glucoheptonate): to detect acute pyelonephritis (risk for scarring) / scar; VUR poses twice the risk of cortical defects than without VUR
CILIOPATHY
= DISORDER OF PRIMARY CILIA (cilia line ducts of kidney + liver and are integral to proper renal + hepatic development
1. Autosomal recessive polycystic kidney disease
2. Autosomal dominant polycystic kidney disease
3. Medullary cystic disease
4. Multicystic kidney disease
5. Meckel-Gruber syndrome
6. Joubert syndrome
7. Orodigitofacial syndrome
8. Asphyxiating thoracic dysplasia
9. Chondroectodermal dysplasia
10. Short-rib polydactyly syndrome
11. Sensenbrenner syndrome (cranioectodermal dysplasia)
12. Weyers acrofacial dysostosis (nail anomalies, polydactyly, oral-facial defects)
GAS IN URINARY TRACT
A. RENAL EMPHYSEMA = renal / perirenal gas
1. Emphysematous pyelonephritis
2. Emphysematous pyelitis
3. Gas-forming perinephric abscess
4. Perinephric emphysema
B. BLADDER
1. Emphysematous cystitis
C. TRAUMA
1. Penetrating trauma
2. Ureterosigmoidostomy, ileal conduit, catheterization with vesicoureteral reflux, percutaneous procedure
CAVE: anomalous posterior position of colon
3. Infarction of renal carcinoma: therapeutic / spontaneous
D. FISTULA TO URINARY TRACT
Connection: bronchus / cutis / GI tract (colon > duodenum > stomach > small bowel > appendix)
1. Inflammation: chronic purulent renal infection, diverticulitis, Crohn disease
2. Neoplastic: colonic carcinoma
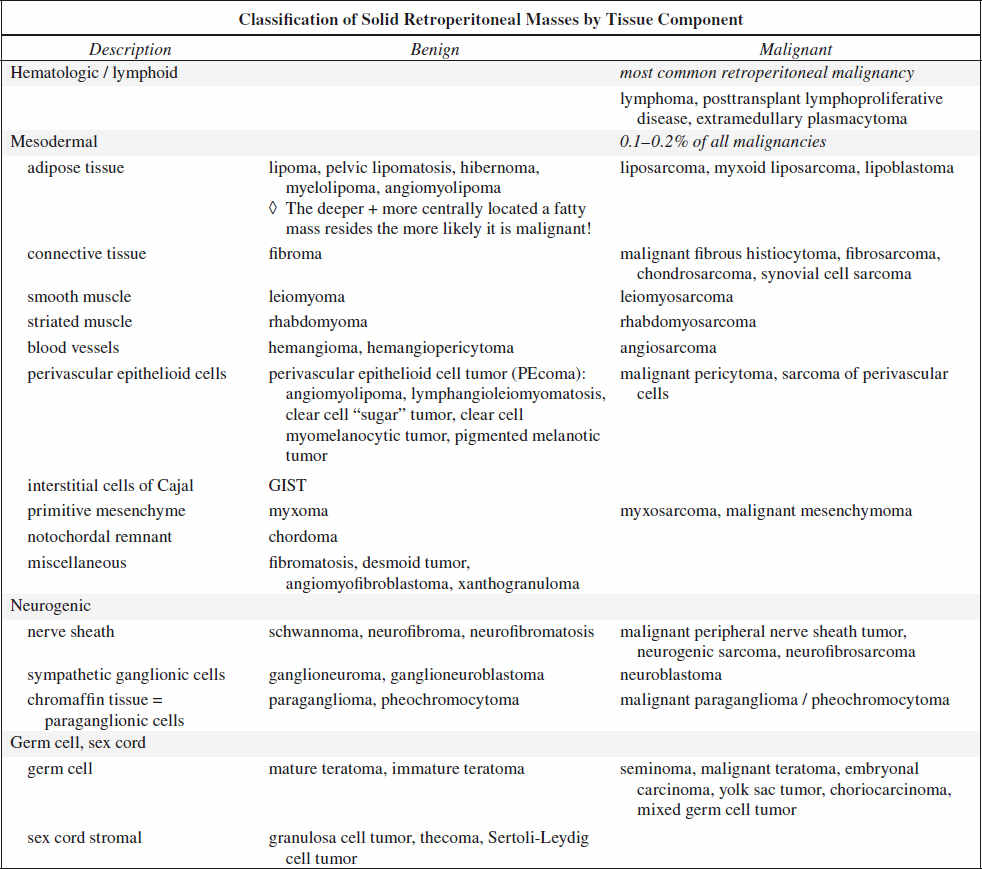
RETROPERITONEUM
70–80% of primary retroperitoneal neoplasms are malignant accounting for 0.1–0.2% of all malignancies in the body.
Primary Malignant Tumor of Retroperitoneum
1. Lymphoma most common retroperitoneal malignancy (33%)
2. Retroperitoneal liposarcoma most common primary retroperitoneal sarcoma (33%)
√ contrast enhancement of thick irregular nodular septa
3. Leiomyosarcoma
2nd most common primary retroperitoneal sarcoma (28%)
A retroperitoneal mass with extensive necrosis and contiguous involvement of a vessel is highly suggestive of a leiomyosarcoma.
4. Malignant fibrous histiocytoma 3rd most common primary retroperitoneal sarcoma (19%)
√ dystrophic calcifications in 25%
Mantle-like Soft-tissue Mass Surrounding Aorta
1. Lymphoma
√ homogeneous mass with irregular lobular margins
√ “floating aorta” sign (= anterior displacement of aorta)
2. Retroperitoneal fibrosis
√ tethering of ureters + IVC
3. Erdheim-Chester disease
√ perinephric fibrosis + bone lesions
Cystic Retroperitoneal Masses
A. NEOPLASTIC
1. Cystic degeneration of solid neoplasm:
› paraganglioma
› schwannoma (neurilemmoma)
» ancient schwannoma
› leiomyosarcoma
2. Cystic teratoma
3. Retroperitoneal lymphangioma (1% of all retroperitoneal neoplasms)
4. Lymphangiomatosis
5. Lymphangiomyomatosis
6. Cystadenoma / cystadenocarcinoma: mucinous / serous
7. Cystic mesothelioma
B. CYSTS:
1. Müllerian cyst
• obese woman under hormonal replacement therapy
2. Epidermoid cyst
Location: midline presacral
√ unilocular
3. Tailgut cyst
4. Pseudocyst
• Hx of acute pancreatitis, elevated amylase level
C. NONNEOPLASTIC: hematoma, urinoma, lymphocele
Cystic Mass with Areas of Solid Enhancement
1. Myxoid liposarcoma
2. Schwannoma
3. Neurofibroma
Neurogenic tumors are common along sympathetic ganglia in the paraspinal region and in adrenal medulla / organ of Zuckerkandl (paraaortic bodies). Less commonly neurogenic tumors occur in urinary bladder, abdominal wall, bowel wall, or gallbladder.
Cystic Mass with Slowly Progressive Enhancement
1. Lymphangioleiomyoma
2. Urinoma
Low-density Retroperitoneal Mass
1. Lipoma
√ sharply marginated, homogeneously fatty mass
2. Lymphangioma
√ similar to lipoma if enough fat content
3. Renal angiomyolipoma
4. Adrenal myelolipoma
5. Xanthogranulomatous pyelonephritis
6. Metastatic retroperitoneal tumors
8. Fibrosarcoma, fibrous histiocytoma, mesenchymal sarcoma, malignant teratoma
√ density close to muscle
9. Retroperitoneal liposarcoma
10. Lipoblastoma
11. Hibernoma
Fat-containing Presacral Mass
1. Lipoma
2. Lipomatosis
3. Liposarcoma: infiltrative growth
4. Teratoma: younger individual
5. Myelolipoma: elderly patient
The differentiation of presacral myelolipoma from other fat-containing lesions may not always be possible with imaging alone.
Heterogeneous Fat-containing Retroperitoneal Mass
1. Dedifferentiated Liposarcoma
2. Myelolipoma
√ positive for erythroid elements on 99mTc-sulfur colloid
3. Angiomyolipoma
Vascular Retroperitoneal Mass
A. INFLAMMATORY
B. LYMPHOPROLIFERATIVE
1. Lymphoma
2. Unicentric Castleman disease
C. MESENCHYMAL TUMOR
1. Sarcoma
2. Solitary fibrous tumor
3. Leiomyoma
4. GIST
5. Hemangiopericytoma
6. Congenital pelvic AVM
D. OTHERS
1. Carcinoid
2. Retroperitoneal paraganglioma
Calcified Retroperitoneal Mass
A. NONTUMORAL
1. Exuberant callus formation
2. Posttraumatic calcified hematoma
3. Myositis ossificans
5. Encapsulated textiloma / gossypiboma
(gossypium, Latin = cotton)
Cause: retained surgical sponge, gauze, towel
B. BENIGN NEOPLASM
1. Ganglioneuroma
2. Schwannoma
3. Paraganglioma
4. Hemangioma
5. Mature teratoma
C. MALIGNANT NEOPLASM
1. Malignant fibrous histiocytoma
2. Dedifferentiated liposarcoma
3. Leiomyosarcoma
4. Malignant mesenchymoma
5. Malignant teratoma
6. Extraskeletal osteosarcoma
7. Chondrosarcoma
8. Ewing sarcoma
Retroperitoneal Fluid
1. Traumatic injury to: pancreas, duodenum, renal collecting system
2. Retroperitoneal hemorrhage
3. Hypoperfusion shock complex
4. Abdominal compartment syndrome
5. Resuscitation effect
Disorders of Perirenal Space
A. PRIMARY LESION
(a) benign
1. Extramedullary hematopoiesis
2. Extraadrenal myelolipoma
3. Castleman disease
4. Erdheim-Chester disease
(b) malignant
1. Lymphoma / leukemia
2. Metastasis (lung)
B. RENAL LESION WITH EXTENSION
(a) benign
1. Angiomyolipoma
2. Xanthogranulomatous pyelonephritis
3. Leiomyoma
4. Hemangioma
5. Lymphangioma
(b) malignant
1. Renal cell carcinoma
C. RETROPERITONEAL LESION WITH EXTENSION
(a) BENIGN
1. Retroperitoneal fibrosis
(b) MALIGNANT
1. Lymphoma / leukemia
2. Metastatic lymphadenopathy
3. Malignant fibrous histiocytoma
4. Liposarcoma
5. Plasma cell neoplasm
Solitary Perirenal Mass
A. benign
1. XGP
2. Castleman disease
3. Hemangioma
4. Leiomyoma
B. malignant
1. RCC
2. Malignant fibrous histiocytoma
3. Lymphoma / leukemia
C. BORDERLINE
1. Hemangiopericytoma
2. GIST
Rindlike Perirenal Mass
1. Lymphoma
2. Retroperitoneal fibrosis
3. Erdheim-Chester disease
Multiple Perirenal Masses
1. Metastases: malignant melanoma; cancer of lung, breast, prostate
2. Plasma cell neoplasm: multiple myeloma, plasmacytoma, plasma cell leukemia
Fat-containing Perirenal Mass
1. Angiomyolipoma
2. Retroperitoneal liposarcoma
3. Extramedullary hematopoiesis
4. Extraadrenal myelolipoma
ADRENAL GLAND
Functioning Adrenal Mass
1. Cushing syndrome and Cushing disease
2. Primary aldosteronism
3. Pheochromocytoma
Adrenal Medullary Disease
1. Neuroblastoma
2. Ganglioneuroblastoma
3. Ganglioneuroma
4. Pheochromocytoma
Adrenal Cortical Disease
1. Adrenocortical hyperplasia
2. Adrenocortical adenoma
3. Adrenocortical carcinoma
4. Cushing syndrome
5. Conn syndrome
6. Adrenogenital syndromes
Adrenocortical Hyperfunction
1. Adrenogenital syndrome
2. Conn syndrome = hyperaldosteronism
3. Cushing syndrome = hypercortisolism
Bilateral Adrenal Masses
1. Metastases (50%)
2. Lymphoma (50% of secondary lymphomas, 50% bilateral)
3. Adenoma (20%)
4. Pheochromocytoma (10%)
5. Myelolipoma (5–13%)
6. Adrenocortical carcinoma (2–6%)
7. Hemorrhage
8. Granulomatous infection (usually bilaterally asymmetric)
9. Hyperplasia (usually bilaterally symmetric)
mnemonic: 4 H PM
Hodgkin disease
Hyperplasia
Hemorrhage
Histoplasmosis / TB
Pheochromocytoma
Metastasis
Granulomatous Disease
Organism: tuberculosis, histoplasmosis, blastomycosis
√ usually bilateral homogeneous adrenal enlargement in acute phase
√ sometimes cystic / calcified adrenals in chronic phase
Cx: tuberculous adrenal atrophy → adrenal hypofunction → Addison disease
Incidental Unilateral Adrenal Mass = Incidentaloma
= incidental discovery of a clinically silent adrenal mass of > 10 mm in a patient without known cancer
Prevalence: 4–9% of all CT exams (in 0.2% of patients 20–29 years, in 7% of elderly); 1.0–4.2% of population
Concern for: primary adrenal cortical carcinoma, metastasis, Cushing disease, pheochromocytoma, aldosteronoma versus nonfunctioning adenoma
◊ With a Hx of malignancy an adrenal nodule is a metastasis in merely 26–36%!
◊ 97–98% of incidentalomas are benign and insignificant!
◊ Most adenomas can be accurately characterized by NECT + MR ± CECT
◊ Follow-up imaging has a limited role
◊ Biopsy only if CT + MR do NOT indicate adenoma / PET-CT suggests metastasis
mnemonic: PLAN My HAM
Pheochromocytoma (6%)
Lymphoma
Adenoma (71%): functioning (1%)
Neuroblastoma
Myelolipoma
Hemorrhage
Adenocarcinoma (4%)
Metastasis (2–3%)
Bilaterality of Adrenal Incidentaloma
Frequency: in < 30% of adenomas
DDx: metastasis, lymphoma, infection, hyperplasia, hemorrhage
Morphologic Criteria of Adrenal Incidentaloma
(a) Size of lesion (too unreliable as only criterion)
Principle: Larger lesions are more likely malignant and more likely symptomatic!
› with known malignancy
< 3 cm: 87% are benign
> 3 cm: 95% are malignant
› without known malignancy
> 4 cm: 70% are malignant
> 6 cm: 85% are malignant
Rx: excision for incidentaloma > 5 cm
(b) Change in size
◊ Any increase in size after 6 months can be considered malignant!
CAVE: rare adenomas + myelolipomas can slightly increase in size
(c) Margin of lesion
◊ Irregular borders usually indicate malignancy!
◊ Multinodularity is usually benign!
(d) Internal texture
◊ Large necrotic areas signify malignancy
◊ Small metastases are often homogeneous
Problematic features of any adrenal mass: diffusely heterogeneous attenuation / focal areas of low attenuation / thickened wall
LIPID-SENSITIVE IMAGING OF ADRENAL INCIDENTALOMA
Principle: a benign adrenal mass contains intracytoplasmic fat, a malignancy does not
Substrate: cholesterol, fatty acids, neutral fat
Distribution: 70% of adenomas are lipid-rich; 30% of adenomas are lipid-poor + indeterminate
NECT attenuation (HUNECT):
◊ dependent on scanner type + scanning technique!
ROI: in center of mass covering at least 50% of cross-sectional area
≤ 10 HUNECT = benign lipid-rich adenoma / cyst
> 10 HUNECT = indeterminate / lipid-poor adenoma
Sensitivity for adenomas: 56–71%
Specificity for adenomas: 98%
False-positive rate: 4%
For all practical purposes a < 3 cm homogeneous lesion that measures ≤ 10 HU at NECT is an adrenocortical adenoma!
CT histogram for NECT / CECT:
√ > 10% of pixel count below 0 HU indicates adenoma (sensitivity of 71% for NECT, 12% for CECT)
Chemical shift imaging MR (CSI):
Physics: fat protons precess at lower frequency than water protons
Useful: if lesion < 30 HU on NECT
(a) qualitatively (and effective):
√ fat + water summate to intermediate SI on in-phase images
√ fat + water signals cancel each other out to a low signal intensity on out-of-phase images
Most adenomas show a significant decrease in signal intensity on the out-of-phase images!
(b) quantitative method (rarely used in practice)
√ adrenal-to-spleen SI ratio (SIR) < 0.71
SIR = SIRin-phase ÷ SIRopposed-phase
√ signal intensity index (SII) > 16.5%
SII = (SIin-phase – SIopposed-phase) ÷ SIin-phase x 100
Results: on average 67% of all indeterminate adrenal adenomas > 10 HU are lipid rich on CSI:
100% of adenomas measuring 10–20 HU
75% of adenomas measuring 20–30 HU
13% of adenomas measuring ≥ 30 HU
Indeterminate: lipid-poor adenomas with low lipid-to-water proton ratio per voxel
PERFUSION IMAGING OF ADRENAL INCIDENTALOMA BY CT
= CT washout scan (most effective test)
Principle: malignant vessels have an increased capillary permeability with prolonged retention of contrast material
Input values:
» 60-second scan (HUCECT 1 min)
» 15-minute delayed scan (HUCECT 15 min)
» ± precontrast CT (HUNECT)
(a) Relative Percentage Washout (RPW) (if unenhanced CT not available)
= [HUCECT 1 min – HUCECT 15 min] / [HUCECT 1 min] • 100%
washout > 60% = lipid-poor adenoma
washout ≤ 60% = indeterminate mass
Results: 96% sensitive + 100% specific
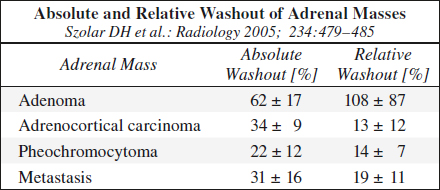
(b) Absolute Percentage Washout (APW) (if unenhanced CT available)
= [HUCECT 1 min – HUCECT 15 min] / [HUCECT 1 min – HUNECT] • 100%
washout > 40% = lipid-poor adenoma
washout ≤ 40% = indeterminate mass
Results: 88% sensitive + 96% specific
Lesions with RPW of < 40% / APW of < 60% are almost always malignant!
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree