Technical Aspects of Abbreviated Breast MRI
For abbreviated breast MRI, it is important to choose a pulse sequence protocol that is easy to run, that is reliable and robust, and that yields reproducible results. The results published on the utility of AB-MRI have been achieved with the image acquisition technique described in the following. 1
We use a four-element surface coil that allows ample access to the breast and thus allows adequate positioning of the breast in the coil. Our current abbreviated protocol consists of three dynamic frames (one pre-, two postcontrast acquisitions), and a quick T2-weighted sequence. Details are given in ▶ Table 4.1.
Any motion of the breast during the acquisitions or between the pre- and the postcontrast acquisition must be meticulously avoided. This is best achieved by usingfixation plates e.g. by Noras Medical Systems, Germany. One should use fixation plates that immobilize the breast ( ▶ Fig. 4.1) in the slice encoding direction. For axial bilateral imaging, this is the craniocaudal direction; for sagittal imaging, this is the mediolateral direction ( ▶ Fig. 4.2).
In a screening situation, both breasts must be imaged simultaneously. Irrespective of the available MR methods, this is best achieved in the axial plane. Bilateral imaging in the sagittal plane takes about double the number of sections to cover two breasts compared with axial imaging. Accordingly, for AB-MRI for screening, axial orientation is greatly preferable. Axial bilateral imaging also facilitates the categorization of non–mass-like enhancement because it provides a side-to-side comparison of both breasts.
Regarding the choice of pulse sequence, we use two-dimensional (2D) multislice gradient echo acquisitions. For the selective depiction of enhancing tissue, we prefer not to apply fat suppression, but image subtraction, for several reasons.
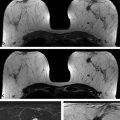
First, fat is an important “contrast agent” for breast imaging; it is indeed the only “contrast agent” available for radiographic breast imaging (mammography or for digital breast tomosynthesis [DBT]). In mammography, a cancer is depicted and its margin status can be evaluated only if it is surrounded, at least in part, by fat tissue. The transparent fat tissue provides the contrast that is needed to visualize a cancer’s morphologic details. Similarly, MR images that are obtained without fat suppression, that is, with fat signal preserved, provide important architectural information that can be used to characterize enhancing lesions based on their architectural features, or based on the effects they have on the architecture of the adjacent normal fibroglandular tissue ( ▶ Fig. 4.3; ▶ Fig. 4.4; ▶ Fig. 4.5; ▶ Fig. 4.6; ▶ Fig. 4.7; ▶ Fig. 4.8). The level of information of a precontrast, high-resolution, non–fat-suppressed T1- or T2-weighted turbo spin echo (TSE) image is at least equivalent to that of a set of DBT images ( ▶ Fig. 4.3d; ▶ Fig. 4.6d; ▶ Fig. 4.7f; ▶ Fig. 4.8c). For instance, fine details of the growth pattern of a lesion with respect to Cooper’s ligaments are best evaluated if the fat signal is preserved ( ▶ Fig. 4.6d; ▶ Fig. 4.7c; ▶ Fig. 4.8c). Moreover, retaining the signal from fat is helpful for further categorization of some enhancing lesions such as fat necrosis ( ▶ Fig. 4.5). Especially breast radiologists who are used to reading mammograms will greatly appreciate the wealth of architectural information that is available if no fat suppression is used, and will be intuitively able to use this information.
Fat suppression will therefore diminish the diagnostic information that is attainable through MR imaging. Fat signal should not be considered a source of “noise,” but it does provide important diagnostic information that can be exploited for image interpretation. 2
Second, fat is an important contributor to the overall MR signal. Active fat suppression substantially lowers the signal-to-noise ratio (SNR) of a given MR image. This is even more problematic given the fact that breast MRI acquisition protocols need to combine fast and high resolution imaging, and are usually acquired by employing parallel imaging. Each of these factors will reduce the resulting image SNR. High resolution imaging implies imaging with very small voxels that contain only a small amount of protons; fast imaging implies imaging with only a single signal average; parallel imaging like SENSE, GRAPPA, or ASSET are associated with another 30% SNR penalty.
Third, active fat suppression takes extra acquisition time; the time needed for active fat suppression may interfere with the temporal resolution (i.e., acquisition speed) and/or the spatial resolution that is achievable. Both, high spatial resolution and fast acquisition techniques per se may already result in borderline (low) SNR of breast MRI; if active fat suppression is added, such protocols may be relatively noisy, thus limiting the detection of subtle enhancing areas.
Fourth, active fat suppression may not work reliably for large-field-of-view (FOV), bilateral breast imaging. Yet for screening, both breasts must be imaged synchronously before and after contrast. This is best achieved by axial imaging with a FOV that is large enough to cover both breasts (not the chest or the upper arm, though). Typical sizes of FOVs will be around 290 to 350 mm. It is challenging to achieve an absolutely homogeneous magnetic field across such large FOVs, especially since the patient herself will interfere with the field homogeneity. Inaccurate fat suppression is problematic for many reasons; for instance, field inhomogeneities may lead to inadvertent water suppression and thus suppression of cancer signal.
Fifth, active fat suppression leads to the fact that tissues with short T1 relaxation time(e.g. proteinaceous fluid), will exhibit bright signal already before contrast injection. Precontrast bright signal caused by fat suppression means that actively fat suppressed images do also require image subtraction for the selective visualization of enhancing tissue (i.e., tissues that are bright because of contrast-agent-induced T1 shortening, as opposed to tissues that are bright due to short T1 secondary to proteinaceous fluid). Accordingly, actively fat suppressed images do require image subtraction anyway.
For all these reasons, our pulse sequence protocol does not apply active fat suppression, but subtraction. Since we do not use fat suppression, it is essential to adhere to in-phase echo time settings; this is 4.6 ms for 1.5 T and 2.3 ms for imaging at 3.0 T. With regard to field strength, we still prefer 1.5-T imaging over 3.0 T imaging for breast MRI. The theoretical advantage of 3.0 T is its impact on the overall MR signal; compared to 1.5-T imaging, 3.0 T should increase the SNR. However, in our practical experience, these advantages more or less reside in theory. Physical properties associated with 3.0 T imaging such as strong dielectric effects, B1 inhomogeneities leading to variable B1 fields (flip angles achieved) and thus T1 contrast, as well as sensitivity to all sorts of artifacts (pulsation, susceptibility), and the problem of tissue heating (radiofrequency absorption) are all difficult to tackle. There are, of course, methods available to deal with these difficulties, but all of them will be associated with an SNR penalty or reduce image acquisition speed that will, in turn, offset the theoretical SNR advantage. Accordingly, we prefer 1.5-T imaging for breast, and even more so for AB-MRI. 3
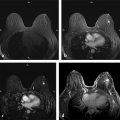
For contrast-enhanced subtracted (non–fat-suppressed) MRI at 1.5 T, we prefer 2D multislice to 3D gradient echo ( ▶ Fig. 4.9). This is despite the fact that based on MR imaging physics, 2D should have a number of disadvantages compared with 3D, as follows:
Multislice 2D imaging is associated with a TR (repetition time) that will range between 250 and 300 ms, depending on the number of sections. Compared to 3D gradient echo imaging, this is a relatively long TR, that is, less strong T1 contrast, and thus less sensitivity to contrast-agent-induced effects.
Moreover, compared to 3D, section thickness of 2D gradient echo acquisitions will be much thicker. Isotropic acquisitions, that is, acquisitions where the section thickness is as small as the pixel dimension in-plane are unattainable with 2D gradient echo. Rather, the pixels or voxels of 2D gradient echo acquisitions will be greatly “un-isotropic,” that is, exhibit a high in-plane resolution, with pixel sizes of 0.5 × 0.5 mm true (noninterpolated) acquisition, but a lower through-plane resolution, with section thickness of about 3.0 mm.
In spite of these theoretical advantages of 3D versus 2D imaging, in clinical practice, for non-fat-saturated contrast enhanced MRI, 2D gradient echo imaging is greatly preferable. In our experiences, this is because of the following: 3D acquisitions are called 3D because phase encoding is used for spatial resolution in all three planes, whereas with 2D, there is a slice-selective excitation. Accordingly, in 3D acquisitions, phase errors can arise in all three dimensions. This can lead to significant image blurring. In 2D pulse sequences, contours of anatomic structures are much sharper. Micromotion during image acquisition will contribute to these contour-blurring effects in 3D acquisitions. To evaluate morphological details of tumor margins or internal architecture, however, all blurring, pulsation artefacts and other types of image degradation must be meticulously avoided. In our practical experience, this is best achieved with 2D gradient echo acquisitions. One should remember that wherever elsewhere in body and neuro MRI, the focus is on evaluating structure, margins, and architecture for differential diagnosis of lesions, radiologists rely on un-isotropic 2D acquisitions, usually even spin echo based.
It should be noted that the section thickness should not exceed 3 mm because otherwise partial volume averaging effects may in turn interfere with our ability to assess contours of small (≤3 mm) enhancing lesions.
In our clinical practice, the fact that 2D gradient echo is associated with longer TRs than 3D gradient echo is indeed advantageous. 2D imaging is a more “conservative” pulse sequence in that it does not aggravate enhancement. It “downplays” enhancement, especially of normal fibroglandular tissue. Since enhancement of benign changes with 2D gradient echo will be less “compelling” or suspicious, we made the experience that the PPV of breast MRI is better with 2D compared with 3D gradient echo imaging — at least if a protocol without fat saturation or fat suppression is employed. Since background enhancement can interfere with the utility of maximum intensity projection (MIP) images, using a “conservative” pulse sequence that does not overemphasize benign background enhancement is of key importance for AB-MRI.
Since enhancement of benign changes are difficult to deal with especially in countries with difficult medicolegal climate, using a “conservative” pulse sequence that does not even exhibit enhancement of benign changes appears particularly advantageous.
If 2D multislice gradient echo is used, it is important to note that one should try to use as few sections as possible. This is because with 2D gradient echo imaging, the TR, and thus the acquisition time, will increase with an increasing number of sections. Our technologists are therefore trained to prescribe the image stack of the dynamic series to exactly cover the volume of the fibroglandular tissue (and not fat tissue above or below the fibroglandular tissue volume). Using fixation plates in the craniocaudal direction serve several purposes here because they reduce the volume of the breast in the craniocaudal direction. With such fixation plates, a usual size breast will be covered by between 25 and 31 sections. A collateral benefit of such reduced number of sections is that fewer sections need to be reviewed by the interpreting radiologist.
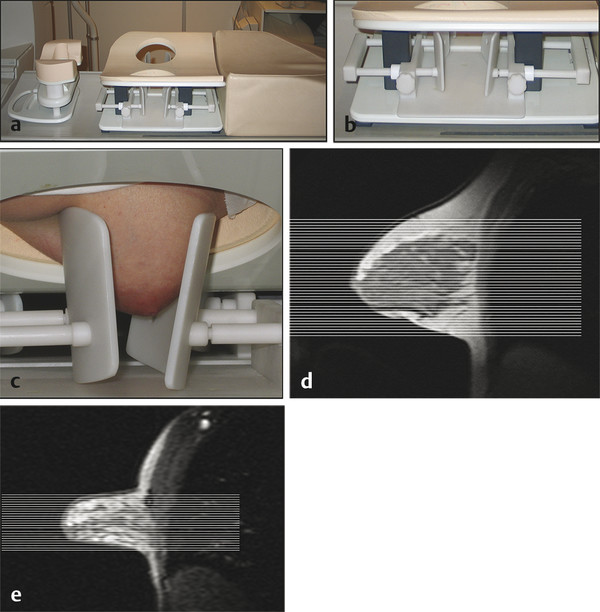
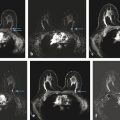
Fig. 4.1 Hardware used. (a) Open breast coil with headrest and breast fixation device in place. The breast coil (in vivo four-channel coil) is open, allowing good access to position the breast. (b) Close-up view of the fixation device. The fixation paddles offer breast compression in craniocaudal direction, which is important for an axial image acquisition (slice-encoding direction). (c) Close-up view with a patient. Note that the breasts are only gently fixated; no forced compression is applied. (d) Sagittal localizer image without breast compression. (e) Sagittal localizer image with breast fixation. Note that less axial sections are needed to cover the fibroglandular tissue.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree