Temporary and Retrievable Inferior Vena Cava Filters
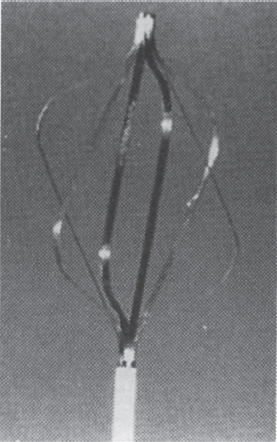
The concept of a removable or nonpermanent filter is not new. As long ago as 1986, Darcy et al1 described successful placement and removal of an Amplatz filter (William Cook Europe, Bjaeverskov, Denmark) (Fig. 40–1) from a patient after 16 days of implantation. In his 1989 review article, entitled “Inferior Vena Cava Filter: Search for an Ideal Device,” Yune2 predicted that if a suitable device could be developed, there would be a significant increase in the use of vena cava filters for temporary applications. Since this article was written, increasing interest has developed in this new class of vena cava filters, and numerous devices are now either available or in the stage of development.3 For clarity of discussion, we prefer to subclassify nonpermanent filters into two broad groups.3
 Definitions
Definitions
Temporary Filters
These are devices attached to a catheter or guidewire that may protrude from the insertion site.
Retrievable Filters
These are permanent filters that have an incorporated design feature that will permit removal of all or possibly part of the device.
 Advantages and Disadvantages of Temporary versus Retrievable Filters
Advantages and Disadvantages of Temporary versus Retrievable Filters
Because temporary filters usually will have an external portion, the patient’s mobility will be limited and the risk of infection increased. Also, temporary devices usually will have to be removed. Retrievable filters have the advantage that they can be left in permanently if necessary. The technique of removal of a retrievable filter, however, such as by snaring a hook, usually will be more technically difficult than removal of a temporary filter and also may involve the use of a costly removal kit.
 Rationale for the Use of Temporary and Retrievable Filters
Rationale for the Use of Temporary and Retrievable Filters
The Patient May Not Require Long-term Protection
In their excellent paper, Ferris et al4 estimated that about 30% of their patients received long-term anticoagulation following filter placement. Mohan et al5 found that anticoagulation was either continued or restarted from several hours to 2 weeks following filter placement in 26% of their 196 patients. Furthermore, Becker et al6 recommended that anticoagulation therapy, when not contraindicated, be used in conjunction with a filter. Consequently, unless the filter was placed for a failure of anticoagulation, it appears that a substantial proportion of patients receiving filters are subsequently anticoagulated and therefore no longer require the filter to protect them from pulmonary embolism.
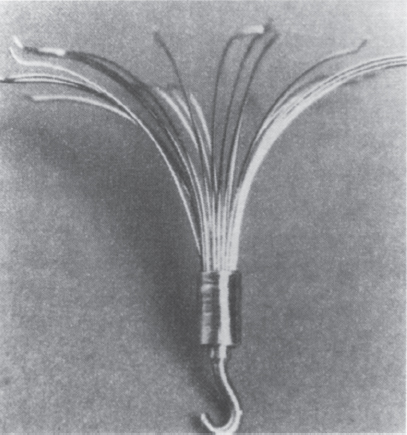
FIGURE 40–1. (a) The Amplatz filter, (b) The device has a hook on its inferior aspect which permits snaring and retrieval from a femoral approach. (Reproduced with permission from Lundt G, Rysavy J, Hunter DW, Castaneda-Zuniga WR, Amplatz K. Retrievable vena cava filter percutaneously introduced. Radiology 1985;195:831.)
Do Filters Provide Long-term Protection Against Pulmonary Embolism?
Decousus et al7 performed a randomized controlled trial to evaluate the safety and efficacy of permanent filters in patients with either pulmonary embolism or proximal deep vein thrombosis (DVT). All patients were treated with anticoagulants in the standard fashion, either intravenous unfractionated heparin or low-molecular-weight heparin, followed by at least 3 months of oral anticoagulant therapy. Graded compression stockings also were prescribed for 3 months. Patients were randomized to receive either a permanent filter or no filter. The filters used were the Vena Tech LGM, titanium Greenfield, Cardial, and Bird’s Nest. All patients underwent baseline and day 12 ventilation–perfusion lung scans. The follow-up scan was performed earlier than day 12 if patients developed symptoms suggestive of pulmonary embolism. Combining symptomatic and asymptomatic pulmonary embolism, they detected a significant reduction (p = 0.03) in the rate of pulmonary embolism in the patients with filters (1% versus 4.8%) At 2 years, however, no difference was seen in the rate of symptomatic pulmonary embolism between the two groups. These data suggest that filters may be most useful in the short term and appear to make a case for a nonpermanent device.
Permanent Filters Increase the Risk of Deep Vein Thrombosis
In the randomized trial described already,7 during the 2-year follow-up period, 12% of the patients in the no-filter group had recurrent venous thrombosis compared with 21% in the patients with filters. In the filter group, 16 of the 37 patients with recurrent venous thrombosis had inferior vena cava (IVC) occlusion. Four of these patients developed symptomatic pulmonary embolism during the follow-up period. Similarly, in the study of Ferris et al4 12 (9%) of the 137 patients with objective IVC imaging had proven symptomatic IVG thrombosis. New or worsening venous thrombosis was demonstrated in 26 (22%) of the 117 patients who received follow-up venous imaging. Mohan et al5 found symptomatic IVC thrombosis in 5% of their patients, and 40% of these patients presented with phlegmasia cerulea dolens. These IVC thromboses developed a median of 26 days following filter placement.
Crochet et al8 estimated IVC patency in 142 patients receiving filters, almost all of whom received objective follow-up imaging. Using Kaplan–Meier analysis, IVC patency was 92% at 2 years, 80% at 4 years, and 70% at 6 years. They also noted that the status of their patients’ lower extremities deteriorated during follow-up, edema being present in 37% at 2 years and 59% at 6 years. Venous trophic disease also was noted to be present in 12% at the time of filter placement but in 53% after 6 years. Crochet et al,8 however, did not demonstrate a relationship between the presence of venous trophic disease and patency of the IVC or use of anticoagulation therapy.
These data suggest that the presence of an IVC filter increases a patient’s risk for subsequent deterioration or recurrence of venous thrombosis. The relationship between this and the presence of IVC occlusion is currently not clear, but IVC occlusion may be a risk factor in its development. Furthermore, because these risks increase with time following filter placement, they could be either improved or avoided by filter removal.
Avoidance of Other Filter-related Complications
A variety of uncommon complications occurring with permanent filters also could be addressed by removal of a temporary or retrievable filter. These include delayed migration, fracture of components, penetration of the IVC, guidewire entrapment, and safety and image quality issues during magnetic resonance imaging.3,4 Concerns about the long-term safety of conventional permanent filters resulted in a hesitancy to place filters in young patients with a long life expectancy.9,10 These concerns also have caused others to urge radiologists to resist broadening the indications for filter placement and to avoid the use of prophylactic filters.11
 Limitations to the Use of Temporary and Retrievable Filters
Limitations to the Use of Temporary and Retrievable Filters
Implantation Time
The maximum implantation period appears to vary markedly, depending on several factors such as animal species being studied, device structure (and possibly material), and technique of removal. In addition, other factors, such as capture of emboli by the device and drug treatment, could influence the rate of development of neointimal hyperplasia and consequently influence the device’s maximum implantation time. Initial studies generally were performed in animals, but subsequently some data became available from human work.
In the subgroup of retrievable filters, both the Amplatz filter (Fig. 40–1) and the Gunther Tulip filter (Fig. 40–2) (William Cook Europe) have reported implantation periods in humans. The Amplatz filter has been retrieved from a patient after an implant period of 16 days.1 The Gunther Tulip filter has been successfully removed from a child after an implant period of 14 days12; however, the maximum safe implantation time for retrievable filters is unknown.
With temporary filters, implant periods have been reported with the Gunther temporary filter (William Cook Europe) (Fig. 40–3), the Antheor filter (Medi-Tech/Boston Scientific, Watertown, MA) (Fig. 40–4) and the Protect infusion catheter (Bard Radiology/C.R. Bard, Covington, GA) (Fig. 40–5). In Europe, a similar device is available and is called the Prolyser filter (Cordis Europe, Roden, The Netherlands). For the Gunther temporary filter, we were able to remove the device after up to 14 days of implantation in all but one of our patients in whom there was firm incorporation of the device into the IVC wall requiring subsequent surgical removal after only 12 days of implantation.3,13,14 Vos et al15 were able to remove this filter successfully in all their patients after up to 14 days of implantation.15 Our incorporated filter was probably infected,14 which may affect the rate of incorporation. These data suggest that the maximum implantation time may vary from patient to patient. Zwaan et al16 reported their experience treating 67 patients with 12 Gunther temporary filters, 11 Protect (Prolyser) devices, and 44 Antheor filters. They do not report the implantation period for each individual device; however, from their data, it appears that they were able to remove the Protect device and the Antheor filter after up to 9 days of implantation.
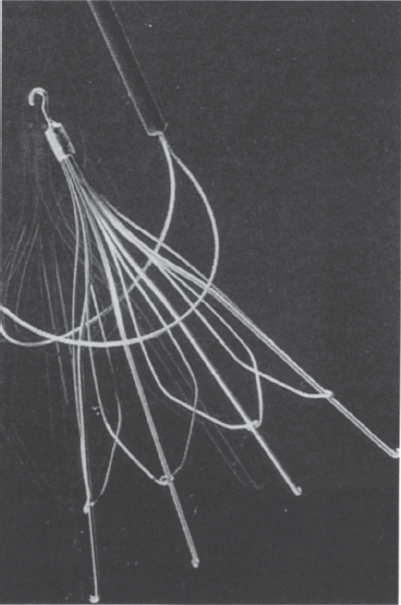
FIGURE 40–2. The Gunther Tulip filter. The filter has a hook on its superior aspect that permits retrieval from a jugular approach. The manufacturers supply a retrieval kit consisting of a snare, which is shown in this figure.
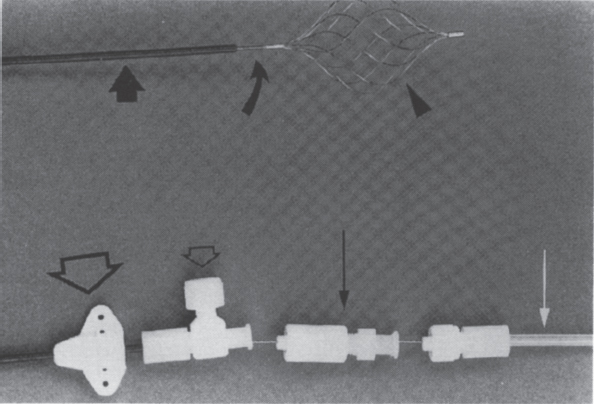
FIGURE 40–3. The Gunther temporary filter. Filter basket (arrowhead), 0.038-inch wire shaft (curved arrow), 6.5-F introduction sheath (large arrow), skin fixation device (large open arrow), flushing port (small open arrow), fixation screw (large straight arrow), and sterility protection tube (white arrow). (Reproduced from ref. 13, with permission.)
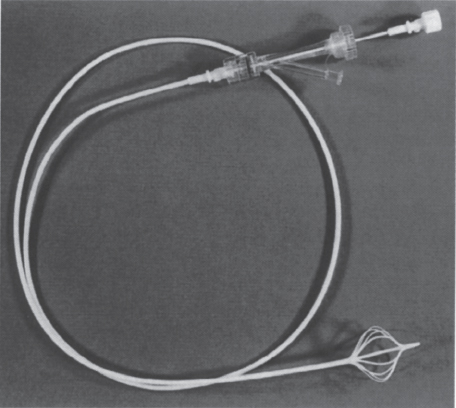
FIGURE 40–5. Protect infusion catheter.
Much longer periods of implantation are possible with the Tempofilter (B. Braun Medical, Evanston, IL) (Fig. 40–6). It is designed for 30 days of implantation but has been removed successfully in humans after up to 55 days of implantation.17 In this series, one patient did require surgical removal after an unspecified implantation period.17 The long implantation period possible with the Tempofilter may be due primarily to the geometry of the filter and the technique of removal. The filter is a cone-shaped device, and only short lengths of the smooth struts at the base of the cone are in contact with the IVC wall. As the filter is removed these smooth struts, which lack hooks or other fixation methods, are slid out from the IVC wall even from organized thrombus adhering them to the IVC wall.18 These factors probably explain the longer implantation periods of the Tempofilter compared with the other designs, in which hooks are torn from the IVC wall or larger components of the filter are in contact with the IVC wall and are removed by a more perpendicularly applied force.
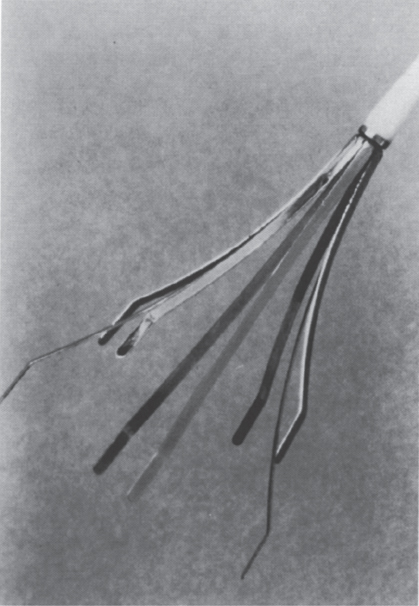
FIGURE 40–6. The Tempofilter. (Reproduced with permission from Millward SF. Temporary and retrievable inferior vena cava filters: current status. J Vasc Intervent Radiol. 1998;9:381–387.)
Problems at the Time of Removal
First, during attempts to remove a nonpermanent filter, resistance to attempted removal may be experienced or removal may be impossible. We encountered this problem with use of a Gunther temporary filter that required subsequent surgical removal.14 In addition, in our experience, a substantial amount of traction is required to remove the Gunther temporary filter in the presence of IVC thrombosis.13 Epstein et al19 were able to retrieve the Amplatz filter from five patients after up to 16 days of implantation; however, they reported that some of the filters were difficult to remove and required complex maneuvers to disengage the partially incorporated filter from the IVC wall. A potential advantage of a retrievable filter over a temporary filter is that if the filter resists removal, it can be left in place, assuming the device is suitable to function as a long-term permanent filter.
Second, the nonpermanent device could be either partially thrombus-filled or there could be extensive IVC thrombosis below the device. Small trapped emboli could be retrieved with the device or possibly allowed to embolize to the lung. Removal of thrombi with the filter has been described with temporary filters.13,15–17 Alternatively, larger trapped thrombi could be treated with thrombolysis,16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree