Fig. 1
Biocontinuum of adverse early and late effects for the testis (with permission From Rubin and Casarett 1968)
2 Anatomy and Histology
2.1 Overview of Normal Gonadal Development
Although the chromosomal and genetic sex of an embryo is determined at fertilization, male and female morphological sexual characteristics do not differ until the seventh week of gestation (Moore 1988). This initial period of early genital development is referred to as the indifferent stage of sexual development. During the fifth week, proliferation of the mesothelial cells and of the underlying mesenchyme produces a bulge on the medial side of the mesonephros, known as the gonadal ridge. Next, finger-like epithelial cords grow into the underlying mesenchyme. The indifferent gonad now consists of an outer cortex and inner medulla (Moore 1988). During the sixth week, the primordial germ cells enter the underlying mesenchyme and incorporate into the primary sex cords. In embryos with an XY sex chromosome complex, testis organizing factor (H-Y antigen) regulated by the Y chromosome determines testicular differentiation (Moore 1988); the medulla differentiates into a testis and the cortex regresses. The gonads can be recognized as testes 7–9 weeks post-fertilization (Figs. 2 and 3a, b).
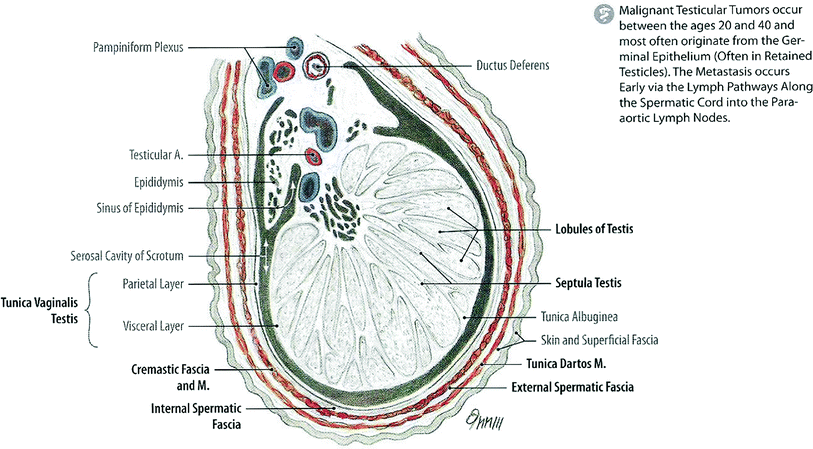
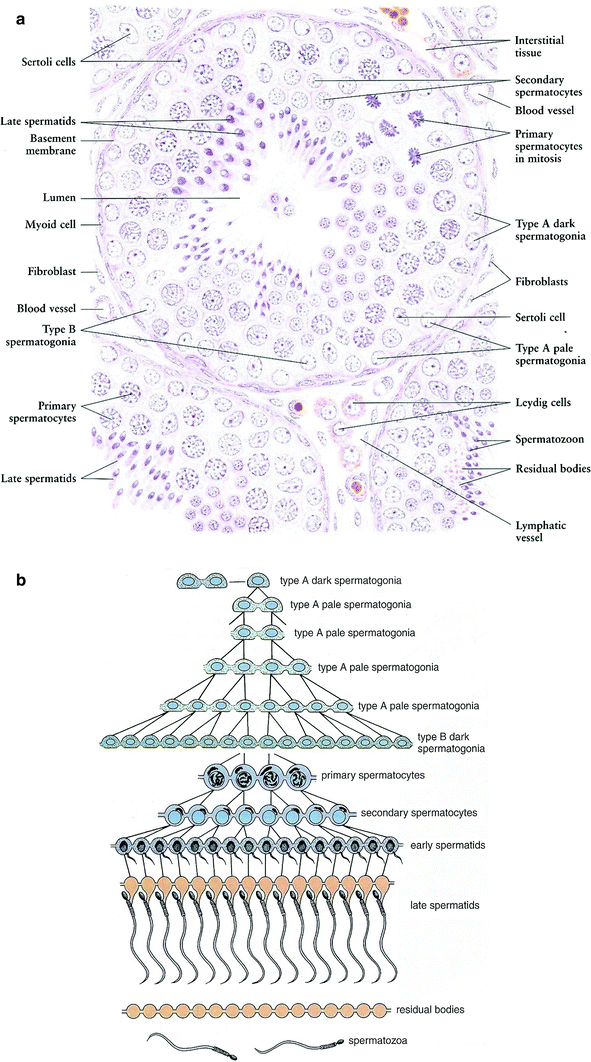

Fig. 2
Anatomy (with permission from Tillman 2007): schematic of a cross section through the testis

Fig. 3
a Microscopic appearance of the seminiferous tubule (with permission from Zhang 1999). b Illustration of spermatogenic cell generations. This diagram shows the clonal nature of the successive generations of spermatogenic cells. Cytoplasmic division is complete only in the primitive type A dark spermatogonia that serve as stem cells. All other spermatogenic cells remain connected by intercellular bridges as they undergo mitotic and meiotic division and differentiation of the spermatids. The cells separate into individual spermatozoa as they are released from the seminiferous epithelium. The residual bodies remain connected and are phagocytosed by the Sertoli cells. (with permission from Ross and Pawlina 2011)
The first stage of testis differentiation is the formation of testicular cords consisting of Sertoli precursor cells packed tightly around germ cells. The diploid germ cells, the prespermatogoonia, undergo meiosis in the fetal testis and remain in meiotic arrest until puberty. Sertoli cells, which provide a location for support and proliferation of spermatogonia are derived from the mesonephros and proliferate only during fetal life and in the neonatal period (Styne 1982). After the 8th week of fetal life, the Leydig cell of the fetal testis secretes testosterone resulting in masculinization of the external genitalia and urogenital sinus. By the third month, the penis and prostate form (Conte and Grumbach 1990). Normal testes descend by the 7th month of gestation with little likelihood of continuing spontaneous descent after 9 months (Czeizel et al. 1981).
2.2 Anatomy of Normal Testis
The adult testis is an oblong organ, approximately 4.5 cm in length, weighing 34–45 g (Steinberger 1989) (Fig. 2). The testis is composed of three principal cell types: germ cells that develop into sperm, Sertoli cells that support and nurture developing germ cells and are also the site of production of the glycoprotein hormone inhibin, and Leydig cells that are responsible for testosterone synthesis (Sklar 1999). Seminiferous tubules, the sites of spermatogenesis, are formed by germ cells and Sertoli cells (Fig. 3a). The Leydig cells that are responsible for the production of testosterone lie near the basal compartment of the seminiferous tubules, enabling them to deliver high concentrations of testosterone necessary for normal spermatogenesis and male secondary sexual characteristics (Morris 1996; Sklar 1999) (Fig. 2).
The seminiferous tubules are embedded in a connective tissue matrix containing interspersed Leydig cells, blood vessels, and lymphatics and are surrounded by a basement membrane (tunica propria) upon which the seminiferous epithelium rests. Spermatogenesis takes place in the seminiferous epithelium. The least differentiated germ cells, the spermatogonia, divide to form spermatocytes that immediately undergo meiosis (Fig. 3b). The haploid cells (the spermatids) that are formed develop into flagellate motile spermatozoa. This process requires up to 74 days (Smith and Rodgriguez-Rigau 1989). Since spermatozoa are continuously produced in adult men, a constant supply of germ cell precursors is necessary. The newly formed spermatozoa are transported through the lumen of the seminiferous tubules into the epididymis where they are stored.
3 Biology, Physiology, and Pathophysiology
3.1 Hypothalamic-Pituitary-Testicular Axis
The primary regulators of testicular function are the anterior pituitary hormones, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), both of which are released in response to the hypothalamic releasing factor, GnRH (Fig. 4). GnRH is secreted from the median eminence into the hypophyseal portal system in a pulsatile manner and acts on the gonadotropes of the pituitary gland to stimulate secretion of LH and FSH (Morris 1996). LH regulates Leydig cell function by binding to specific LH receptors on the plasma membrane of Leydig cells. This leads to the formation of the cAMP that drives testosterone biosynthesis via a complex cascade starting with cholesterol (Morris 1996; Sklar 1999). Testosterone is transported from Leydig cells to the seminiferous tubules, where it acts to enhance spermatogenesis (Sklar 1999). Testosterone is also a prohormone for two different and metabolically active hormones, dihydroxytestosterone (DHT) and estradiol. DHT mediates male sexual differentiation and virilization, whereas estradiol mediates bone maturation, mineralization, and epiphyseal fusion (Smith et al. 1994). Testosterone controls pituitary LH secretion by a negative feedback mechanism; LH levels will rise when the Leydig cells are unable to produce testosterone (Figs. 4, 5a, b, Tables 1, 2, 3).
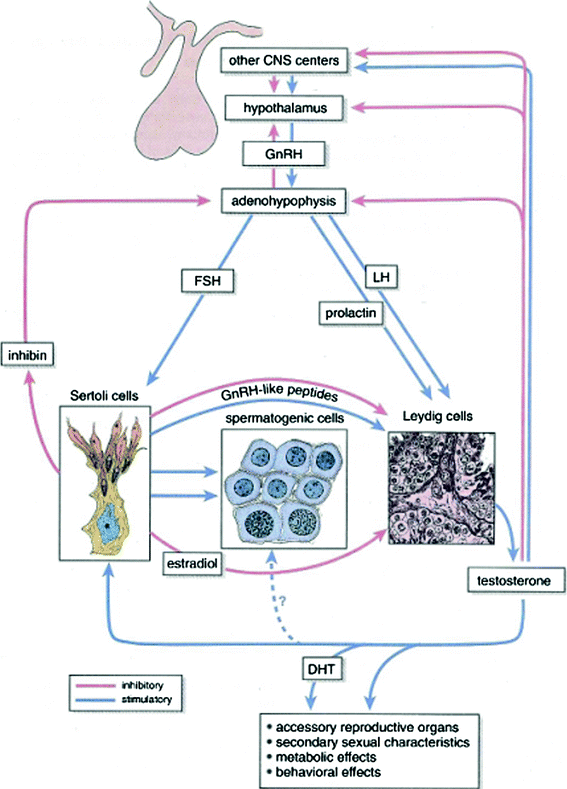
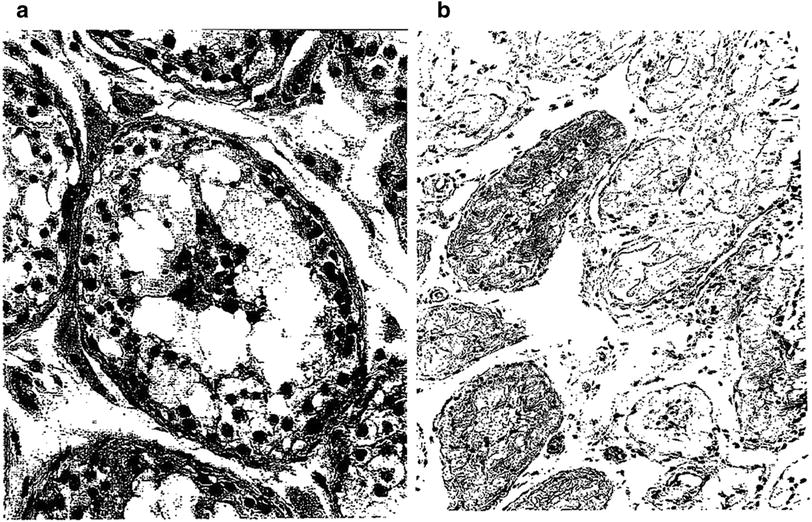

Fig. 4
Hormonal regulation of the testis (Blue arrows) indicate stimulatory action on the system; (red arrows) indicate inhibitory feedback (with permission from Ross and Pawlina 2011)

Fig. 5
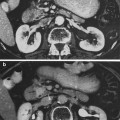
a Early necrosis of germ cells of the type observed hours to a few days after administration of large single doses of radiation. In comparison with the normal germ cells, there is a marked decrease in the layers of germ cells, absence of spermatozoa, and groups of necrotic cells in the center of the lumen. The stroma is not altered. b Late testicular atrophy: this 60 year old man received an estimated dose of cGy of fractionated radiation to both testes during irradiation pelvis and perineum in the course of therapy for carcinoma of the prostate. Notice, one year later, the convoluted basement membrane in the midst of thick tubular walls. In this very advanced degree of testicular atrophy, Sertoli cells are no longer present in most tubules…tubular lumina are either empty of contain “foam cells” (with permission from Fajardo 2001)
|
Stage
|
Description
|
Mean age at onset (year)
|
|---|---|---|
|
(Range 95 %)
|
||
|
1
|
Preadolescent. Testes, scrotum, and penis are about same size and proportion as in early childhood
|
|
|
2
|
Scrotum and testes have enlarged; change in texture and some reddening of the scrotal skin. Testicular length > 2 cm < 3.2 cm
|
11.6
|
|
(9.5–13.8)
|
||
|
3
|
Growth of penis has occurred, at first mainly in length but some increase in breadth; further growth of testes and scrotum. Testicular length > 3.3 cm < 4.0 cm
|
12.9
|
|
(10.8–14.9)
|
||
|
4
|
Penis further enlarged in length and breadth with development of glans. Testes and scrotum are further enlarged. Scrotal skin has darkened. Testicular length > 4.1 cm < 4.9 cm
|
13.8
|
|
(11.7–15.8)
|
||
|
5
|
Genitalia are adult in size and shape. No further enlargement takes place after stage 5 is reached. Testicular length > 5 cm
|
(14.9)
|
|
(12.7–17.1)
|
|
Stage
|
Description
|
Mean age at onset (year)
|
|---|---|---|
|
(Range 95 %)
|
||
|
1
|
Preadolescent. No pubic hair
|
|
|
2
|
Sparse growth of long, slightly pigmented downy hair, straight or only slightly curled appearing chiefly at base of penis
|
13.4
|
|
(11.2–15.6)
|
||
|
3
|
Hair is considerably darker, coarser, and curlier and spreads sparsely
|
13.9
|
|
(11.9–16.0)
|
||
|
4
|
Hair is now adult in type but area it covers is still smaller than most adults. There is no spread to medial surface of the thighs
|
14.4
|
|
(12.2–16.5)
|
||
|
5
|
Hair is adult in quantity and type, distributed as an inverse triangle. The spread is to the medial surface of the thighs
|
15.2
|
|
(13.0–17.3)
|
Table 3
Impairment of spermatogenesis and Leydig cell function after fractionated radiotherapy (Ash 1980)
|
Testicular dose (cGy)
|
Effect on spermatogenesis
|
Effect on Leydig cell function
|
|---|---|---|
|
<10
|
No effect
|
No effect
|
|
10–30
|
Temporary oligospermia
|
No effect
|
|
30–50
|
Temporary azoospermia at 4–12 months after radiation. 100 % recovery by 48 months
|
No effect
|
|
50–100
|
100 % temporary azoospermia for 3–17 months after radiation
|
No effect
|
|
Recovery begins at 8–26 months
|
||
|
100–200
|
100 % azoospermia from 2 months to at least 9 months. Recovery begins at 11–20 months
|
Transient rise in LH
|
|
No change in testosterone
|
||
|
200–300
|
100 % azoospermia beginning at 1–2 months. May lead to permanent azoospermia. If recovery takes place, it may take years
|
Transient rise in LH
|
|
No change in testosterone
|
||
|
1200
|
Permanent azoospermia
|
Elevated LH. Some patients may have decreased basal testosterone or in response to HCG stimulation. Replacement therapy not needed to ensure pubertal changes in most boys
|
|
2400
|
Permanent azoospermia
|
Elevated LH. Many patients, but not all, will have decreased testosterone. Replacement therapy needed to ensure pubertal changes in most boys
|
Sertoli cells are the only cell type of the testis that possess FSH receptors (Sklar 1999). FSH is delivered to the interstitial area of the testis by way of the arterial system. It then passes through the basement membrane of the seminiferous tubule to reach the Sertoli cells that line this membrane. FSH binds to the FSH receptors of the Sertoli cell and then mediates the synthesis of a variety of proteins and enzymes including the inhibins (Morris 1996). Inhibin is a major feedback regulator of FSH and may thus be useful as a marker of spermatogenesis (Hayes et al. 1998; Schmiegelow et al. 2001). The production of androgen transport protein by Sertoli cells can be induced and maintained by FSH.
FSH triggers an event in the immature testis that is essential for the completion of spermatogenesis. Thereafter, spermatogenesis will proceed continuously as long as an adequate and uninterrupted supply of testosterone is available. The lack of negative feedback from germinal epithelium results in an elevated FSH level.
3.2 Normal Developmental Stages
A neonatal surge in testosterone secretion is caused by high LH and FSH concentrations that occur during the first few months of life. From the age of about 3 months until the onset of puberty, plasma concentrations of LH and FSH are quite low and the testes are relatively quiescent (Sklar 1999). At the onset of puberty, pulsatile secretion of LH (and to a lesser extent, FSH) occurs during sleep that is associated with increased nighttime plasma concentrations of testosterone (Sklar 1999). As puberty progresses, the increased pulsatile release of gonadotropins and the high concentrations of testosterone are maintained throughout the day and night (Sklar 1999) (Tables 1, 2).
In a normal male the first sign of puberty is enlargement of the testis to greater than 2.5 cm (Styne 1982). This is due to seminiferous tubule growth, although Leydig cell enlargement contributes as well. Androgens synthesized in the testes are the driving force behind secondary sexual development, although adrenal androgens also play a role in normal puberty. The range of onset of normal male puberty extends from 9 to 14 years of age. Boys complete pubertal development in 2–4.5 years (mean 3.2 years) (Styne 1982). The development of the external genitalia and pubic hair has been described in stages by Marshall and Tanner (1970) (Tables 1 and 2). The first appearance of spermatozoa in early morning urinary specimens (spermarche) occurs at a mean age of 13.4 years, at gonadal stages 3 and 4 (pubic hair stages 2 through 4) simultaneous with the pubertal growth spurt.
3.3 Pathophysiology: Cytotoxic Effects of Therapy
3.3.1 Testicular Irradiation
Soon after the discovery of X-rays by Roentgen, investigators noted that spermatogenesis is exquisitely sensitive to radiation (Albers-Schonberg 1903; Regaud and Blanc 1906). The testes are directly irradiated in rare situations such as testicular relapse of acute lymphoblastic leukemia (ALL). Although the testes are usually not directly in the radiation field, they can still receive irradiation via body scatter. Scatter occurs when X-rays interact with tissues near the target of interest, resulting in secondary X-rays that then hit the target (Khan 2003). The amount of scattered radiation is a function of the proximity of the radiation field to the target, the field size and shape, the X-ray energy, and the depth of the target. Of these, distance from the field edge is the most important factor. Scatter dose to the testes becomes a real issue when treating a field that extends into the pelvis as in some cases of Hodgkin’s disease, seminoma or soft tissue sarcoma of the thigh. Small children, because of their shorter trunk length, can be at greater risk from scattered radiation than larger individuals (Table 3, Fig. 5a, b).
The germinal epithelium is most sensitive to radiation and some effect on spermatogenesis will be seen at doses of 10 cGy (Table 3 shows radiation dose response, and Fig. 5a, b shows early and late histopatholgic correlates) (Ash 1980). Permanent sterilization may be seen with doses as low as 100 cGy (Ash 1980). Speiser et al. reviewed experimental data in mammals that indicated that the total radiation dose required to induce permanent azoospermia was lower if a fractionated regimen was used than if a single dose was given (Speiser et al. 1973). The details of these experiments cannot be extrapolated to humans because there are significant differences in germ cell radiosensitivity between different species. However, the general conclusion that fractionated radiation is more efficient than single doses in destroying germ cells appears to be true in humans as well.
To fully understand, the paradox of fractionated doses of radiation being more efficient than a single dose of radiation, one needs to appreciate the cell cycle kinetics of the testis germinal tissue. The spermatogenic germinal epithelium undergoes 4 cycles within 64 days and is more radiosensitive when the intermitotic interval is short (Spermatogonia type A). As the intermitotic interval lengthens (Spermatogonia B) the cells become less radiosensitive. Once mitosis is completed meiosis begins and the spermatids reduce their chromosomes to a haploid number and form spermatozoa which are very radioresistant.
Classically, a single large dose of radiation is more effective and efficient ablating the cancer rather than fractionating the dose. Fractionation allows for the 4 R’s: repair, reoxygenation, reassortment, and repopulation of the tissue. Thus the total fractionated dose is greater than a single large dose to achieve the same degree of cell kill i.e. the Strandquist lines apply to normal tissues and tumors.
As noted in the “Law of Bergonie and Tribondeau” experiments, small fractionated doses to the testes are more effective and efficient than a large equivalent single dose for testicular sterility to occur without scrotal skin injury. These experiments can be considered the search for radiation oncology’s equivalent to the “Holy Grail”. That is, there is an optimal fractionation dose schedule which can ablate tumors without causing significant damage to normal tissues.
Extreme fractionation of dose enhances cell kill of the testis stem cell population in which “dose-time relationships are such that there is a maximum degree of mitosis linked deaths with a minimum of wasted radiation”. Casarett reproduced these results in experiments in Beagle dogs (Casarett 1964). That is, small doses 3 cGy per week given in small daily doses of 0.6 cGy for 5 days a week, to total cumulated doses of 475 cGy, was able to cause complete and permanent aspermia where as a single large dose of 2000 cGy failed to achieve this endpoint. As state, the population of spermatogonia comprises two subpopulations, spermatogonia A and B, which differ in duration of the intermitotic interval. The most effective fractionation schedule to achieve complete and permanent sterilization of seminiferous epithelium efficiently kills the Type A spermatogonia subpopulation with the shorter intermitotic period and initiates each spermatogenic cycle with more frequent mitoses. Thus, spermatogonia A is more likely to be irradiated during mitosis resulting in depletion of stem cell population.
More attention has been focused on the effects of radiation on spermatogenesis than on its effects on Leydig cell function. The data available, indicates that chemical changes in Leydig cell function are observable following direct testicular irradiation with the effect more pronounced with 2400 cGy than with 1200 cGy (Sklar et al. 1990). The severity of the effect is more marked the younger the patient at the time of radiotherapy (Castillo et al. 1990). Sertoli cell function may also be affected at doses of 3000 cGy (Tsatsoulis et al. 1990). In general, progression through puberty and testosterone production proceeds normally in males subjected to radiation therapy.
3.3.2 Chemotherapy
Testicular dysfunction is among the most common long-term side effects of chemotherapy in men. The germinal epithelium is particularly susceptible to injury by cytotoxic drugs secondary to a high mitotic rate. Leydig cells in contrast, appear relatively resistant to the effects of chemotherapy (Tsatsoulis et al. 1990). In 1948, azoospermia after an alkylating agent (nitrogen mustard) was described in 27 of 30 men treated for lymphoma (Spitz 1948) Subsequently, it has become apparent that all alkylating agents are gonadotoxic (Heikens et al. 1996; Mackie et al. 1996; Papadakis et al. 1999). Antimetabolite therapy in general (e.g., methotrexate, mercaptopurine) does not have an adverse impact on male fertility. Cisplatin-based regimens result in temporary impairment of spermatogenesis in all patients but with recovery in a significant percentage (Hansen and Hansen 1993).
Initial reports suggested that the immature testis was relatively resistant to chemotherapy. More recently, however, it has become apparent that both the prepubertal and pubertal testes are vulnerable to cytotoxic drugs (Ben Arush et al. 2000; Hobbie et al. 2005; Howell and Shalet 1998). Impairment of spermatogenesis may be irreversible in the months to years following chemotherapy. However, late recovery of spermatogenesis up to 14 years following chemotherapy has been reported (Buchanan et al. 1975; Watson et al. 1985). In summary, testicular damage is agent-specific and dose-related. The chance of recovery of spermatogenesis following cytotoxic chemotherapy and the extent and speed of recovery are related to the agent used and the dose received (da Cunha et al. 1984; Howell and Shalet 2001; Pryzant et al. 1993; Watson et al. 1985). In contrast to the germinal epithelium, Leydig cells appear relatively resistant to the effects of chemotherapy (Shalet et al. 1989). However, a few studies have demonstrated a reduction in testosterone concentrations following treatment with gonadotoxic agents, and there is evidence to suggest that the Leydig cell impairment following chemotherapy may be clinically relevant.
4 Clinical Syndromes
The data for gonadal function following fractionated radiotherapy in humans comes from patients with cancers who have been treated with either fields near the testes in which there is scatter dose or diseases such testicular cancers or ALL in which the testes are thought to be at risk of harboring disease and irradiated directly (Fig. 6 and Table 4).
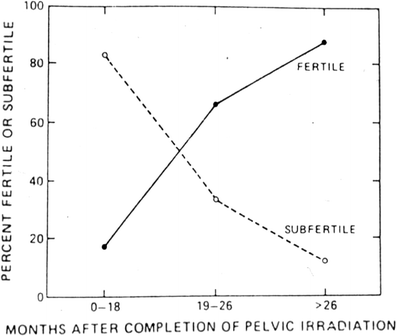

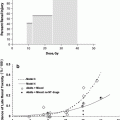
Fig. 6
Recovery of spermatogenesis following pelvic irradiation for Hodgkin’s disease (with permission from Pedrick and Hoppe 1986)
Table 4
LENT SOMA toxicity scoring for testicular injury from radiation
|
Grade 1
|
Grade 2
|
Grade 3
|
Grade 4
|
|
|---|---|---|---|---|
|
Testes
|
||||
|
Subjective
|
||||
|
Libido
|
Occasionally suppressed
|
Intermittently suppressed
|
Persistently suppressed
|
|
|
Objective
|
||||
|
Fertility
|
Oligospermia
|
Azoospermia
|
||
|
Appearance
|
Atrophy
|
|||
|
Management
|
||||
|
Fertility
|
In vitro fertilization
|
Sperm retrieval if previously banked
|
||
|
Libido
|
Testosterone
|
|||
|
Analytic
|
||||
|
FSH/LH
|
Increased FSH/nl LH
|
Increased FSH/increased LH
|
||
|
Testosterone
|
Decreased
|
|||
|
Spermatoanalysis
|
Assessment of sperm number, motility and morphology
|
|||
|
Flow cytometric analysis
|
Assessment of sperm chromatin integrity
|
|||
|
DNA chromatin analysis
|
Assessment of sperm chromatin integrity
|
|||
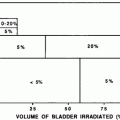
Ash summarized data from several older studies (Hahn et al. 1976; Sandeman 1966; Speiser et al. 1973) that examined testicular function following radiation for patients who were treated for a range of cancers including Hodgkin’s disease, prostate cancer, and testicular cancer (Ash 1980). They found that oligospermia occurred at doses as low as 10 cGy and azoospermia at 35 cGy, which was generally reversible. However, 200–300 cGy could result in azoospermia that did not reverse even years after irradiation.
A decrease in sperm counts can be seen 3 to 6 weeks after irradiation, and, depending on the dosage, recovery may take 1–3 years (Fig. 6). The germinal epithelium is damaged by much lower dosages (<1 Gy) of radiation than are Leydig cells (20–30 Gy). Complete sterilization may occur with fractionated irradiation above doses of 2–4 Gy.
These results have been supported by other reports. In a study of 17 males treated for Hodgkin’s disease who received 6–70 cGy of scatter dose to the testes, in those patients who received <20 cGy, FSH levels stayed within the normal range (Kinsella et al. 1989). Those receiving >20 cGy had a dose-dependent increase in serum FSH levels with a maximum difference seen at 6 months following radiation. In all patients the FSH normalized within 12–24 months. Eight patients had normal pretreatment sperm counts. Most of these patients continued to have high sperm counts following irradiation although two developed transient oligospermia with complete recovery of sperm count by 18 months. Four patients, all of whom had received 23 cGy or less, went on to father children. Ortin et al. reported on children treated for Hodgkin’s disease (Ortin et al. 1990). Seven boys received pelvic radiation as part of their treatment without any chemotherapy. Three of them fathered children. Three had azoospermia 10–15 years after irradiation, and one had testicular atrophy diagnosed by biopsy a year after irradiation. However, these results are difficult to interpret because there was no measurement or estimate of dose received to the testes in these children.
Similar data exist for patients treated for soft tissue sarcomas (median age at diagnosis 49 years; range 14–67) (Shapiro et al. 1985). These patients were estimated to have received between 1 and 2500 cGy (median dose 80 cGy) of scatter dose to the testes. Patients who received 50 cGy or more to the testes had a greater elevation in FSH than did those who received less than 50 cGy. Only the latter group showed normalization of FSH levels by 12 months whereas in the former, FSH levels remained above baseline as long as 30 months out.
There are also data on germ cell function after treatment for testicular cancer. Hahn et al. examined gonadal function in 14 patients who were irradiated to the paraaortic and ipsilateral pelvic/inguinal lymphatics with a “hockey stick” field following orchiectomy for seminoma (Hahn et al. 1982). The scatter dose to the remaining testicle in these 14 patients ranged from 32 to 114 cGy (median 82 cGy). Ten patients, all of whom received >70 cGy to the testes, developed azoospermia between 10 and 30 weeks following radiation. All patients except for two had recovery of spermatogenesis, and the recovery time from azoospermia was dose dependent. Centola et al. studying males with seminoma also showed that the recovery time from oligospermia/azoospermia was dose-dependent (Centola et al. 1994).
The previous data includes only patients who received incidental irradiation to the testes; however, there are situations in which children receive direct irradiation to the testes. Sklar et al. examined testicular function in 60 long-term survivors of childhood ALL (Sklar et al. 1990). All the patients had received identical chemotherapy; however, the RT fields varied significantly: (1) craniospinal radiation and 1200 cGy to the abdomen and testes (n
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree