Chapter 20 Testicular Germ Cell Tumors
Introduction
Testicular cancer is the most common malignancy in males ranging from puberty to the fourth decade of life.1
Although the focus of this chapter is on testicular GCTs, it is important to provide an initial overview of GCTs, which include EGGCTs. The most common sites of EGGCTs are the mediastinum, retroperitoneum; less common sites include the pineal gland and sacrococcygeal area. EGGCTs stem from primordial germ cells that failed to migrate properly during embryogenesis. Retroperitoneal GCTs are rarely EGGCTs because most are metastases from a primary testicular tumor. Primary retroperitoneal EGGCTs may be due to an occult gonadal GCT or metachronous primary retroperitoneal and testicular tumors. Both GCTs and EGGCTs share common histopathology and are divided into seminomatous and nonseminomatous types. Both GCTs and EGGCTs also share a similar pattern of age distribution in the 30s.2,3
Testicular GCTs are highly curable, with a 5-year survival rate of more than 95%.1 The death rate from testicular cancer in the United States is less than 400/yr.1,4
Epidemiology and Risk Factors
The incidence of GCTs in the United States from 2001 to 2005 was 11.8 per 100,000.1 Approximately 8400 new cases of testicular GCT are diagnosed in the United States each year.1 The incidence is age-dependent: rare before age 5, most common at age 20 to 39, then declining at age 40 onward.5 When the tumors occur in childhood, they predominantly occur in the 4 years and younger age groups.
The incidence in the 20- to 40-year-old age group has doubled over the last three decades.6 From 1976 to 2005, there was an increase of unknown etiology in the incidence in testicular cancer of 1.5% to 2.3%.7–9 Although testicular cancer is common in young males, it still is a rare tumor, estimated at 1% of male malignancies.6 Testicular cancer is the most common solid malignancy in males between 15 and 40 years of age. Therefore, if a man in his late 40s presents with a testicular mass, other diagnoses such as lymphoma or metastases should be considered. Most are unilateral, with approximately 1% to 5% presenting with synchronous or metachronous bilateral tumors.10,11
Testicular cancer is presumed to have a genetic basis. It has a low incidence in black Americans, with a 4.5 times higher prevalence in whites.12 The highest rates are in western and northern Europe and New Zealand.13 Intermediate rates are in the United States.8,14
Risk Factors
Virtually all histopathologic types of GCT have an abnormality in chromosome 12. Postpubertal tumors are aneuploid abnormalities and associated with a gain of the short arm of chromosome arm 12p, which is labeled as i(12p).15 Tumors that stem from prepubertal gonads tend to be diploid abnormalities.5,15–17
Associated risks of developing testicular cancer include intratubular germ cell neoplasia (ITGCN), which is testicular carcinoma in situ. If it is not diagnosed and treated, approximately half of all cases will progress to an invasive malignancy.11,18
Testicular microlithiasis can be a risk factor and is further discussed in the “Ultrasound” section.
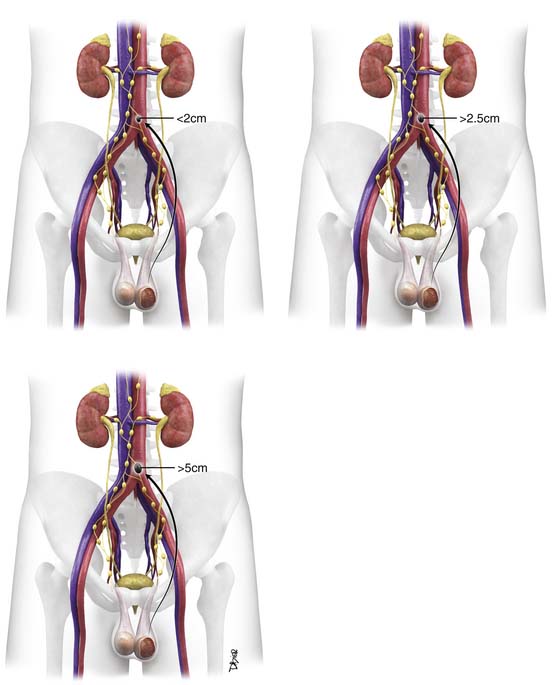
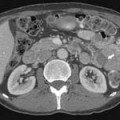
Cryptorchidism is a major risk factor, increasing the risk of germ cell neoplasia 5 to 10 times.19 It is one of the main risk factors that predicts disease occurrence in the testis. The higher the location of the undescended testes, the higher the risk; an abdominal undescended testes has a higher risk than an inguinal undescended testes20 (Figure 20-1). Therefore, it is important to follow-up postorchidopexy patients with a scrotal US. Seminoma accounts for 60% of GCTs with cryptorchidism. The risk factor of developing cancer from cryptorchidism also depends on genetic, environmental, and hormonal factors.19
Another important risk factor is personal past history. Presence of GCT in one testis increases the risk of involvement of the other testis. The 15-year risk of developing a contralateral testicular cancer in affected patients is approximately 1.2%.The incidence of simultaneous bilateral tumors is 1% to 5%.10
Familial history of testicular cancer is a risk factor. Approximately 2% of affected males have a family history, with siblings having up to a 10-fold risk and sons having up to a 6-fold increased risk.21,22
Other associated risk factors are testicular dysgenesis syndromes, which include hypospadias, testicular atrophy, and hypogonadism. Fetal origin testicular cancer is associated with urogenital congenital anomaly, hypospadias, cryptorchidism, and low fetal birthweight.8
Some environmental agents are thought to be associated, including exposure to diethylstilbestrol (DES) during maternal pregnancy, increased estrogen levels in utero,23 and pesticides.24 Other studies have associated increased risk with dietary factors such as maternal smoking, high cheese diet, and body mass index.17,24
Human immunodeficiency virus (HIV) infection is another risk factor of increased testicular cancer.23 Others include Klinefelter’s syndrome, dysplastic nevus syndrome, inguinal hernias, and EGGCTs.5
Key Points Epidemiology and risk factors
• Highest incidence is in males 15 to 44 years old.
• Incidence in patients 20 to 40 years old has doubled over the past three decades.
• Common abnormality is in chromosome 12.
• Cryptorchidism is a major risk factor.
• Other risk factors include environmental, personal and family history, and HIV/AIDS (acquired immunodeficiency syndrome).
Anatomy and Pathology of the Scrotum and Testes
Anatomy
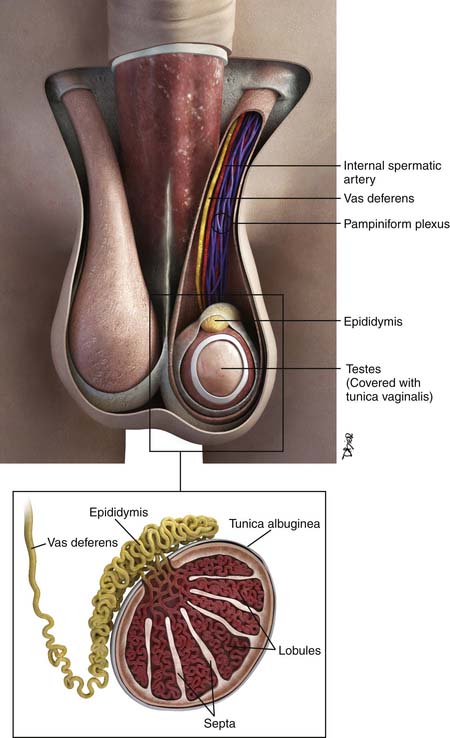
The testes are a component of the scrotum, a pouch that contains the testes, epididymis, and portions of the spermatic cord. Each testis is surrounded by the tunica vaginalis. Surrounding the tunica are fascial layers that compose the scrotal wall. From internally outward, the scrotal wall components are the internal spermatic fascia, the cremasteric muscle, the external spermatic fascia, and the dartos muscle25–27 (Figure 20-2).
Each testis is an elliptical structure containing approximately 400 lobules, which are divided by septa. The septa converge to the mediastum testes, which is contiguous with the tunica albuguinea, a fibrous, dense outer covering of the testis. Each lobule contains two seminiferous tubules, which are lined with germ cells. The seminiferous tubules form a network of ducts known as the rete testis. The ductules connect the rete testis to the head of the epididymis.25–27
The epididymis is composed of a head, body, and tail, from superior to inferior. The body and tail are composed of a single tubule. The tubule joins the vas deferens. The vas deferens courses through the inguinal canal and joins the seminal vesicles to form the ejaculatory duct.25–27
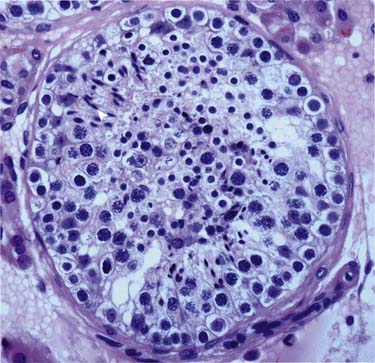
There are several cell types in the testes28:
Germ cells (in seminiferous tubules): The germ cells are the precursors to the spermatogenesis process (Figure 20-3).
Sertoli cells (in seminiferous tubules): Located in the epithelium of the seminiferous tubules and play an important process of germ cells developing into spermatozoa.
Leydig cells (in between seminiferous tubules): Important for puberty development; produce testosterone and other androgens.
Other cells: Immature Leydig cells, epithelial cells, and interstitial macrophages.
Pathology
Tumor Types
The vast majority of testicular tumors are GCTs (95%). GCTs originate from spermatogenic cells. GCTs are believed to originate from tissue stem cells and arise from the germinal epithelium of the seminiferous tubules owing to atypical cell proliferation known as testicular intratubular germ cell neoplasia unclassified (ITGCNU).5,29,30 It is believed that the ITGCNU cells abnormally multiply during puberty, possibly from an altered hormonal environment. The tumors retain stem cell properties, which enable self-renewal that leads to tumorigenesis and differentiation into either seminomatous or nonseminomatous cells, which leads to a varied cellular tumor composition and possible resistance to treatments.17
Typically, germ cells arise from the yolk sac during the fourth gestation week and migrate to the gonadal ridge, iliac fossa, and then the scrotum. When there are abnormalities in the migration of these cells, the various types of GCTs arise. Undifferentiated totipotential stem cells give rise to embryonal carcinoma. If the cells continue to progress toward an embryonic pathway, they become teratomas. If they progress to the extraembryonic pathway, they become choriocarcinomas or yolk sac tumors.31 Figure 20-4 describes the histogenesis of GCTs.17
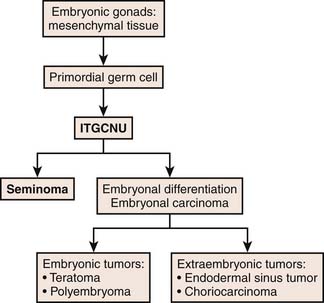
Figure 20-4 Diagram of histogenesis of germ cell tumors.
Redrawn from Cushing B, Perlman E. Germ cell tumors. In: Pizzo PA, Poplack DG, eds. Principles and Practice of Pediatric Oncology. 5th ed. Philadelphia: Lippincott-Raven; 2006.
There are two main classifications of GCTs: seminomas and nonseminomas, which compose approximately 95% of malignant testicular tumors. The remainder are lymphomas, which account for 4%; 1% are rare tumors (e.g., interstitial tumors, embryonal sarcomas, and Sertoli cells).13,32
Gross and Microscopic Features
Seminoma
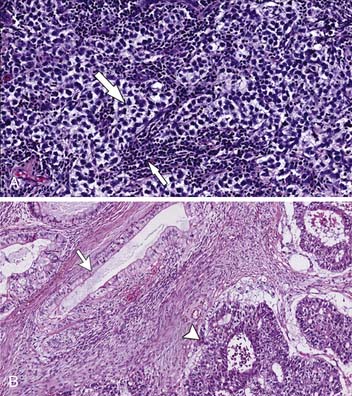
The gross appearance is a homogeneous firm mass with single or multiple nodules. The tumor cells are homogeneous with large round cells with clear cytoplasm in bundles outlined by fibrovascular trabeculae. The bands contain an abundance of plasma cells and T lymphocytes. Granulomatous reaction is common, and hemorrhage and necrosis are rare17,33–35 (Figure 20-5A).
Note that the spermatocytic seminoma is a histologically distinct subtype of seminoma, which is virtually always cured by an orchiectomy and usually no other treatment because it rarely metastasizes.5
Nonseminomatous Germ Cell Tumors
The NSGCTs include a large group of histologically diverse neoplasms such as embryonal, yolk sac tumors, choriocarcinoma, and teratoma. When one or more tumor components are present, they are mixed. The NSGCTs generally are more ill-defined than seminomas and have hemorrhage and necrosis33,34 (see Figure 20-5B).
• Teratoma: Composed of one or more of ectoderm, mesoderm, and endoderm layers. Grossly, it is more cystic and multiloculated, sometimes with cartilage. Sebaceous fat and calcifications are typical findings. Immature teratomas have larger solid components with scattered fat and calcification. Different teratomas contain components of nerve, epithelium, and cartilage. The more mature tumors feature differentiated tissue, and the immature teratomas have fetal-based tissues. Histologically, teratomas are predominantly composed of cystic components, with an epithelial lining similar to the epidermis with some appendages. Approximately 85% contain a solid histologically varied element called Rokitansky’s protuberance.17,33,34,36
• Embryonal carcinoma: Gross appearance is that of an ill-defined mass with hemorrhage and necrosis. Vascular invasion can be present. Histologically, the malignant cells are composed of undifferentiated cells with an indistinct border and an anaplastic epithelial and embryonic appearance. The cells are polygonal with atypia and an elevated mitotic rate, proliferating in a tubular, acinar, solid, or papillary appearance.17,33,34
• Yolk sac tumor: Gross appearance is that of a multilobulated solid mass with a mucinous covering, with possible hemorrhage and necrosis. Histologically, it is composed of primitive tumor cells in a loose reticular pattern. Each cell has hyperchromatic nuclei. A distinguishing feature is a Schiller-Duval body, a fibrovascular core containing single vessels.33,34
• Choriocarcinoma: Grossly a heterogeneous mass, commonly identified with hemorrhage and necrosis. Histologically, it is composed of syncytiotrophoblastic and cytotrophoblastic cells.33,34,36,37
• Mixed tumor: A widely varied composition of histologic subtypes; accounts for up to 60% of testicular GCTs.33,34
It is important to note that the presence of yolk sac elements and undifferentiated cells is an important predictor of tumor relapse.38
Two main classifications of germ tumors are present: the World Health Organization (WHO), commonly used in North America and Europe, and the British Testicular Tumor Panel (BTTP), used in the United Kingdom and Australia. The WHO classification is more common and divides tumor categories into seminomatous and nonseminomatous types. The BTTP divides nonseminomatous tumors into different types of teratomas. Table 20-1 differentiates the WHO and BTTP classifications.17,39
Table 20-1 Comparison of the World Health Organization and the British Testicular Tumor Panel Classifications of Testicular Germ Cell Tumors
| BTTP | WHO |
|---|---|
| Seminoma | Seminoma |
| Spermatocytic seminoma | Spermatocytic seminoma |
| Teratoma | Nonseminomatous germ cell tumor |
BTTP, British Testicular Tumor Panel; MTI, malignant teratoma intermediate; MTU, malignant teratoma undifferentiated; TD, teratoma differentiated; WHO, World Health Organization; YST, yolk sac tumor.
From Chieffi P, Franco R, Portella G. Molecular and cell biology of testicular germ cell tumors. Int Rev Cell Mol Biol. 2009;278:277-308.
Key Points Pathology
• Seminomatous GCTs and NSGCTs together are equally divided in prevalence, and together compose approximately 95% of malignant testicular tumors.
• Seminomas are composed of one histologic type and typically are a homogeneous mass with little necrosis or hemorrhage.
• NSGCTs are composed of one or more histologic type with a more heterogeneous appearance, typically with necrosis and hemorrhage.
Tumor Markers
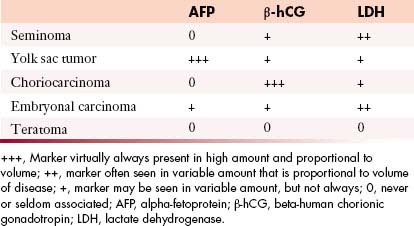
Tumor markers help in the characterization of testicular tumors (Table 20-2).
• Alpha-fetoprotein (AFP): Produced by the fetal yolk sac, gastrointestinal tract, and liver. This tumor marker is elevated in 50% to 60% of patients with NSGCTs such as yolk sac tumors and mixed GCTs containing yolk sac elements.17,40,41
• Human chorionic gonadotropin (hCG): A glycoprotein produced by syncytiotrophoblastic giant cells. It is elevated in 60% of patients with advanced NSCGTs and in 10% to 20% with stage I disease. Advanced seminomatous disease has elevated hCG in up to 25%. Choriocarcinomas (pure trophoblastic teratomas) have very high levels of hCG and metastasize widely.17,40,41
• Lactate dehydrogenase (LDH): A less specific marker because it is produced by multiple organs. It is elevated in greater than 80% of NSCGT cases at the time of initial presentation and is elevated in patients with advanced seminoma. Its levels correlate with the bulk of disease.17,40,41
Clinical Presentation
The most common presentation of testicular cancer patients is a painless testicular mass.42 The clinical presentation is highly variable, such as a palpable testicular mass, pain, and/or swelling. Approximately 10% present with fever and/or scrotal pain.43 Some series have reported that about half of all patients with testicular cancer present with testicular pain with or without a mass. In approximately 20%, symptoms are related to metastatic disease. The patient may also present in the advanced stages with backache from retroperitoneal adenopathy, which should be differentiated from musculoskeletal pain. Other symptoms include neck mass from adenopathy, dyspnea, and hemoptysis from lung metastases or nodal disease in the lungs. Approximately 5% present with gynecomastia. Approximately one in three to four patients presents with abdominal and back pain, headache, malaise, or hemoptysis. The testicular tumor may be an incidental finding secondary to recent trauma in the region, which reveals the finding on further evaluation with imaging or examination.44
If retroperitoneal adenopathy or, less commonly, cervical, supraclavicular, or axillary adenopathy is palpated or detected by US or CT in a male patient, particularly between 15 and 35 years old, a testicular examination and US should be performed to evaluate for an underlying primary tumor.
Patterns of Tumor Spread
Blood Supply of Testes
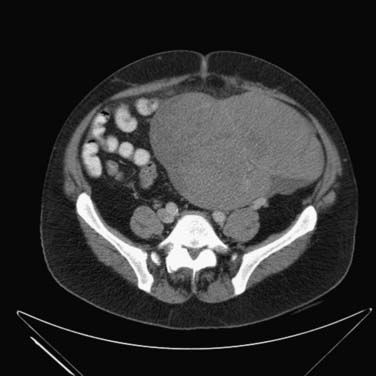
The pair of testicular arteries arises from the aorta. The right testicular artery originates from the abdominal aorta. The left testicular artery originates from the left renal artery. The testicular arteries course through the inguinal canal. The testis features a dual blood supply. These are the cremasteric artery, which is an inferior epigastric artery branch of the external iliac artery, and the artery of the ductus deferens, an inferior vesicle artery branch of the internal iliac artery. The right testicular vein drains into the inferior vena cava (IVC), and the left testicular vein drains into the left renal vein. The lymph node drainage pattern, therefore, predominantly first involves the aortocaval nodes on the right and para-aortic lymph nodes below the renal vasculature on the left.45
Lymphatic Spread
The pattern originates in the mediastinum of the testes, to the internal inguinal ring along the spermatic cords, alongside the lymphatic channels adjacent to the testicular vessels, and to the retroperitoneal lymph nodes.46 This route is based on the embryologic origin of the testes in the retroperitoneum.44
Right-sided nodes have more variability in spread pattern, with preferential spread inferior to the right renal hilum, to the right paracaval, interaortocaval, preaortic, precaval, and retrocaval nodes. Right-sided tumor spreads to right-sided nodes 85% of the time and to contralateral and ipsilateral nodes 13% of the time.13,47,48
Left-sided nodes tend to spread inferior to the left renal vessels to the left para-aortic nodes and preaortic nodes. Left-sided tumor spreads to left-sided nodes 80% of the time and to both contralateral and ipsilateral nodes 20% of the time.13,47,48
The right and left infrarenal periaortic nodes spread to the renal suprahilar nodes and then to the retrocrural nodes. Direct spread can occur through the retroperitoneum to the diaphragm and then to the posterior mediastinal and subcarinal nodes. Indirect spread can occur through the thoracic duct to the prevascular and supraclavicular nodes13,47 (Figure 20-6).
Lymphatic spread can also occur lateral to the aortocaval group to the echeolon node, situated between the first and third lumbar vertebral bodies on the right and anterior to the left iliopsoas muscle on the left.13,49
It is rare to have contralateral nodal metastases without ipsilateral metastatic or primary disease. It is also rare to have direct spread to iliac or inguinal nodes. In the presence of advanced disease, previous scrotal surgery, or cryptorchidism, or at relapse of tumor, pelvic spread and contralateral nodal spread can occur.47 Direct spread to inguinal nodes can occur when there are skin metastases.
Hematogenous Spread
The primary site of hematogenous spread is the lungs.49 Other sites include the brain, which is common with choriocarcinoma. Additional sites include the osseous structures and liver. Rare sites of hematogenous spread include pleura, pericardium, muscle, skin, spleen, kidneys, adrenal glands, and peritoneum.50 Although hematogenous spread is generally associated with synchronous lymph node metastases, it does occasionally “skip” the retroperitoneum in cases of embryonal carcinoma.
Key Points Tumor spread
• Predominant form of tumor spread is through the lymphatics.
• Most common site of metastases is the retroperitoneum, followed by the lungs.
• Direct spread can occur through the retroperitoneum to the diaphragm, then to the posterior mediastinal and subcarinal nodes.
• Indirect spread can occur through the thoracic duct to the prevascular and supraclavicular nodes.
• Hematogenous spread is less common, most often to the lungs.
Staging
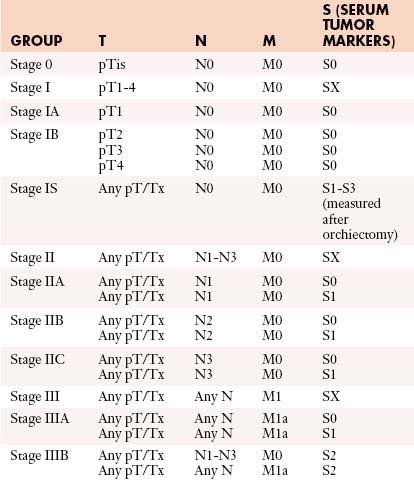
The tumor-node-metastasis (TNM) system is utilized for staging (Figure 20-7).
• Stage I: Disease is confined to the testes with no nodal disease or metastases; consists of IA and IB. IS is persisting elevated tumor markers after orchiectomy.
• Stage II: Disease in the retroperitoneum is confined to lymph nodes. Stage IIA has lymph nodes smaller than 2 cm, stage IIB nodes are 2 to 5 cm, and stage IIC nodes are larger than 5 cm.
• Stage III: Metastases spread beyond the retroperitoneum or to extranodal sites.
Table 20-3 describes the TNM classification of GCTs.51 Table 20-4 lists the staging criteria of GCTs.51
Table 20-3 Tumor-Node-Metastasis Definitions
| Primary Tumor (T)* | |
| The extent of primary tumor is usually classified after radical orchiectomy, and for this reason, a pathologic stage is assigned. | |
| pTX | Primary tumor cannot be assessed |
| pT0 | No evidence of primary tumor (e.g., histologic scar in testis) |
| pTis | Intratubular germ cell neoplasia (carcinoma in situ) |
| pT1 | Tumor limited to testis and epididymis without vascular/lymphatic invasion; tumor may invade into tunica albuginea but not tunica vaginalis |
| pT2 | Tumor limited to testis and epididymis with vascular/lymphatic invasion, or tumor extending through tunica albuginea with involvement of tunica vaginalis |
| pT3 | Tumor invades spermatic cord with or without vascular/lymphatic invasion |
| pT4 | Tumor invades scrotum with or without vascular/lymphatic invasion |
| Regional Lymph Nodes (N) | |
| Clinical | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis with a lymph node mass 2 cm or less in greatest dimension or multiple lymph nodes, none more than 2 cm in greatest dimension |
| N2 | Metastasis with a lymph node mass more than 2 cm but not more than 5 cm in greatest dimension or multiple lymph nodes, any one mass greater than 2 cm but not more than 5 cm in greatest dimension |
| N3 | Metastasis with a lymph node mass more than 5 cm in greatest dimension |
| Pathologic (pN) | |
| pNX | Regional lymph nodes cannot be assessed |
| pN0 | No regional lymph node metastasis |
| pN1 | Metastasis with a lymph node mass 2 cm or less in greatest dimension and less than or equal to five nodes positive, none more than 2 cm in greatest dimension |
| pN2 | Metastasis with a lymph node mass more than 2 cm but not more than 5 cm in greatest dimension; or more than five nodes positive, none more than 5 cm; or evidence of extranodal extension of tumor |
| pN3 | Metastasis with a lymph node mass more than 5 cm in greatest dimension |
| Distant Metastasis (M) | |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| M1a | Nonregional nodal or pulmonary metastasis |
| M1b | Distant metastasis other than to nonregional lymph nodes and lung |
* Note: Except for pTis and pT4, extent of primary tumor is classified by radical orchiectomy. TX may be used for other categories in the absence of radical orchiectomy.
From Testis. In: Edge SB, Byrd DR, Compton CC, eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010:471-472.
Once the tumor is staged, the European Germ Cell Cancer Consensus Group (EGCCCG) recommends that the patient risk be determined using the International Germ Cell Cancer Consensus Group (IGCCCG) guidelines, which is divided into good, intermediate, and poor categories.52,53 The classification is based on tumor location, metastases, tumor markers, and histology. Table 20-5 lists the IGCCCG guidelines.52,53
Table 20-5 International Germ Cell Consensus and Prognosis Classification
| Seminoma |
| Good prognosis: all of the following |
| Intermediate prognosis: all of the following |
| Nonseminoma |
| Good prognosis: all of the following |
| Intermediate prognosis: all of the following |
| Poor prognosis: any of the following |
AFP, alpha-fetoprotein; hCG, human chorionic gonadotropin; LDH, lactate dehydrogenase; N, upper limit of normal.
Adapted from International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594-603.
Poor risk of NSCGT are patients with any one of the following: nonpulmonary visceral metastases such as liver and brain metastases, markedly elevated serum markers (AFP > 10,000 ng/mL or hCG > 50,000 IU/L or LDH > 10 times the upper limit of normal), or mediastinal primary site.54,55
Key Points Staging
• TNM system is widely used to stage testicular tumors. After staging, the IGCCCG guidelines place the patient into good-, intermediate-, and poor-risk categories.
• Stage I is confined to the testis with no nodal disease or metastases.
• Stage II has disease in the retroperitoneum confined to lymph nodes.
• Stage III has metastases that spread beyond the retroperitoneum or to extranodal sites.
Imaging
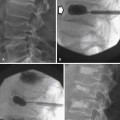
The diagnosis of testicular carcinoma is usually made after an inguinal orchiectomy and is based on the histopathology.56 Percutaneous biopsy of the testicle can lead to seeding along the biopsy tract, so it is never performed if germ cell malignancy is suspected.57
When interpreting radiologic examinations, it is important to note that important predictors of tumor relapse are lymphatic invasion and vascular invasion.38
Ultrasound
US is the primary modality for assessing the testes, with a greater than 95% sensitivity and specificity for detecting testicular lesions. It can be easily performed and is cost-effective. It is used to screen for associated abnormalities such as contralateral disease and microlithiasis. Typically, a high-frequency linear transducer from 7 to 10 MHz is used with imaging in at least two planes.53
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree