Mushabbar A. Syed, MD, FACC
THORACIC AORTIC ANEURYSM
PATIENT STORY
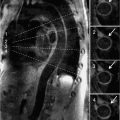
A 69-year-old man with hypertension underwent evaluation for persistent cough. On clinical examination he had an early diastolic murmur best heard at the left sternal border consistent with aortic regurgitation (AR). Chest X-ray demonstrated a widened mediastinum and prominent aortic knob suggesting thoracic aortic aneurysm (TAA) or tortuous aorta. CT scan demonstrated a TAA of the ascending aorta (AA) measuring 4.2 cm without compression of the trachea, bronchus, or esophagus (Figure 8-1). Patient was recommended to follow-up with a subsequent imaging study in 6 months to identify the rate of progression of aortic aneurysm.
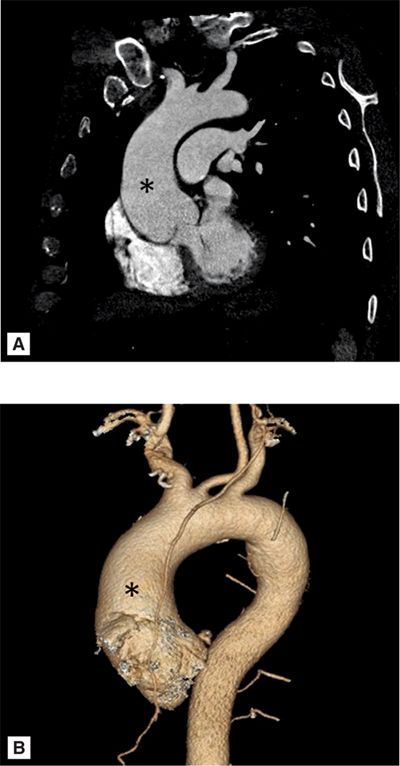
FIGURE 8-1 A 69-year-old man with hypertension underwent evaluation for persistent cough and was found to have a widened mediastinum and prominent aortic knob on chest X-ray. Contrast-enhanced (CE) CT scan demonstrated an ascending thoracic aortic aneurysm (TAA) (A). Three-dimensional reconstruction of the ascending aortic aneurysm is shown (B). * indicates ascending aorta.
EPIDEMIOLOGY
Thoracic aortic aneurysm is defined as dilatation of aorta with a diameter exceeding 1.5 times the expected normal diameter.1 The prevalence of TAAs increases with age. Thoracic aortic aneurysms are less common than abdominal aortic aneurysms (AAAs). The incidence is 6 to 10 cases per 100,000 patient years.2,3 Males are affected 2 to 4 times more commonly than females and occur most commonly in the sixth and seventh decades of life. Hypertension is present in over 60% of patients and is the most important risk factor. Approximately 25% of patients with a TAA also have an AAA.4,5
ETIOLOGY AND PATHOPHYSIOLOGY
A vast majority of TAAs are related to cystic medial degeneration, which leads to weakening of the aortic wall. Histologically, smooth muscle cell dropout and elastic fiber degeneration are seen. These findings are common with advancing age and are accelerated with hypertension.6
Certain genetic conditions lead to premature cystic medial degeneration in young adults. Connective tissue disorders (CTDs) such as Marfan syndrome and Ehlers-Danlos syndrome are genetic defects which predispose patients to premature TAA formation (Figure 8-2). Marfan syndrome is an autosomal dominant disorder that is most often due to a mutation in the fibrillin-1 (FBN1) gene, less commonly the transforming growth factor beta (TGFβ) receptor 2 gene.7 Aortic root disease due to cystic medial degeneration leads to aneurysmal dilatation, AR, and dissection. Fifty percent of children with Marfan syndrome have a dilated aorta and 60% to 80% of adults have aortic root dilatation on echocardiography (ECHO).8,9 In Marfan syndrome, aortic dissection frequently begins just above the coronary ostia and can extend the entire length of the aorta. Marfan syndrome accounted for 4.3% of aortic dissections in one large series.10 Pregnant women with Marfan syndrome are at increased risk of aortic dissection, especially those who have aortic root dilatation.11,12 In addition, the aorta in Marfan syndrome appears to be less distensible with a higher stiffness index measured by magnetic resonance imaging (MRI) compared to controls.13
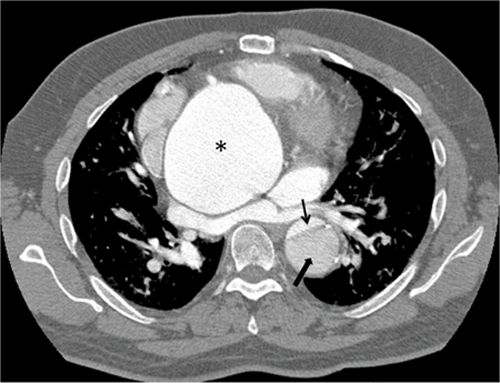
FIGURE 8-2 A 43-year-old man with Marfan syndrome presenting with chest pain found to have a type B dissection on a contrastenhanced CT scan. * indicates ascending aortic aneurysm. Thin black arrow demonstrates the small true lumen. Thick black arrow demonstrates the larger false lumen separated from the true lumen by an intimal flap.
In Ehlers-Danlos syndrome, a defect in type III collagen leads to hypermobility of joints, hyperelasticity of skin, and frequently, mitral valve (MV) prolapse. Aortic root dilatation is uncommon. Spontaneous rupture of large- and medium-sized arteries, usually without dissection, is the most serious cardiovascular complication.
Familial associations with TAAs are found in up to 19% of patients without underlying CTDs such as Marfan syndrome or Ehlers-Danlos syndrome.14 Patients with familial TAA syndrome present at a significantly younger age than those with sporadic aneurysms; most pedigrees suggest an autosomal dominant inheritance.
Traditional atherosclerotic risk factors such as hypertension, smoking, and hypercholesterolemia have been associated with descending TAAs. However, it is uncertain whether atherosclerosis is causal or strictly associated with TAA formation.15,16 Aneurysm formation appears multifactorial in nature leading to defects in vascular structural proteins, with atherosclerosis occurring secondarily. One hypothesis involves the role of proteases such as matrix metalloproteinases, collagenase, elastase, and plasmin in the breakdown of extracellular elastin and collagen. These proteases are derived from inflammatory cells infiltrating the media and adventitia, smooth muscle and endothelial cells, which leads to cystic medial necrosis.17 Proteases accelerate smooth muscle cell necrosis and elastic fiber degeneration with cystic spaces in the media, leading to vessel dilation and ensuing aneurysm formation.17
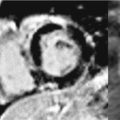
Bicuspid aortic valves are associated with thoracic aortic dilatation, especially of the aortic root and AA in 50% of patients (Figure 8-3).18 Approximately 50% of patients with coarctation of the aorta have a bicuspid aortic valve, which is an independent predictor of aortic aneurysmal formation.19 Aneurysm formation is felt to be secondary to cystic medial degeneration and decreased expression of the fibrillin-1 gene. Bicuspid aortic valve is associated with aortic root dilatation and ascending aortic aneurysms in patients with Turner syndrome.19
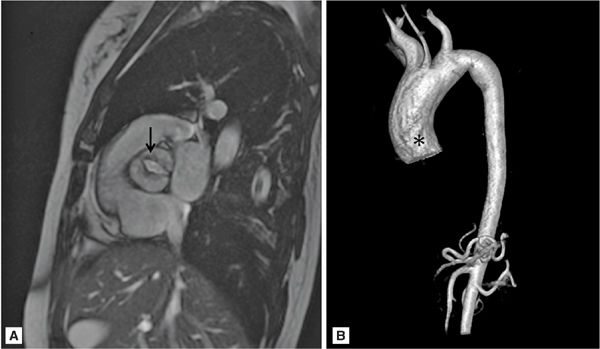
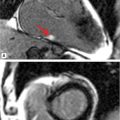
FIGURE 8-3 A 50-year-old man with fatigue and dyspnea on exertion. (A) Short-axis SSFP cine of the aortic valve demonstrates bicuspid aortic valve opening in systole (black arrow). (B) Three-dimensional MR angiography demonstrates an ascending TAA extending to the brachiocephalic artery consistent with aortopathy associated with bicuspid aortic valve. * indicates ascending aorta (AA).
Aortitis presents from a wide array of inflammatory and infectious etiologies which can cause aortic aneurysms. Giant cell arteritis, Takayasu arteritis, and ankylosing spondylitis are most common in the adult population. The pathophysiologic process is felt to be secondary to intramural inflammation and degeneration associated with an infection or an autoimmune process. One of the more common inflammatory causes of TAAs is giant cell arteritis, which occurs in 11% to 18% of patients.20 Aortic aneurysms and dissections are a late manifestation of giant cell arteritis and usually occur in otherwise asymptomatic individuals.21 Takayasu arteritis affects women more often than men; it is usually diagnosed in the third or fourth decade of life. Stenosis of the aorta and other branch vessels is most common (Figure 8-4). In 15% of patients, aortic dilatation occurs resulting in TAAs.
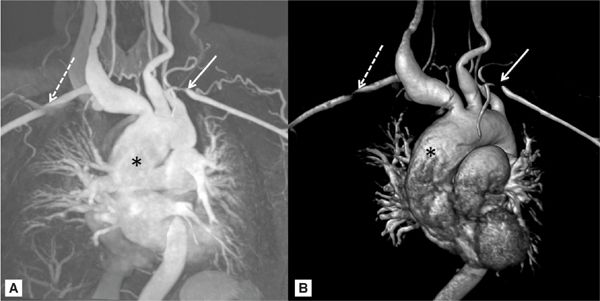
FIGURE 8-4 A 42-year-old asian woman with a pulsatile right-sided neck mass and arm claudication was diagnosed with Takayasu arteritis. (A) MR angiogram and (B) three-dimensional reconstructed MR angiogram demonstrates an ascending TAA (*) with ectasia of all branch vessels originating from aortic arch. White arrows indicate bilateral subclavian artery stenosis.
DIAGNOSIS
A variety of imaging modalities are useful in the diagnosis of TAAs. Often, a chest X-ray incidentally identifies an asymptomatic patient with the characteristic widened mediastinal silhouette, enlargement of the aortic knob, and displacement of the trachea from the midline. However, chest X-rays cannot distinguish an aneurysm from a tortuous aorta and many aneurysms are not apparent on chest X-ray.6 Transthoracic echocardiography (TTE) can visualize the aortic root; but it does not visualize the mid-distal ascending or descending aorta well. Thus, other imaging modalities such as cardiovascular MRI and computed tomography (CT) play a vital role in diagnosis. MR angiography (MRA) or CT angiography (CTA) are the preferred modalities to detect TAAs, determine their size, and to identify anomalies of the aortic arch or branch vessels.22,23 When the aortic root is involved, ECG gating should be used with MRI or CT because it is more accurate in sizing the root diameter by avoiding motion artifacts from nongated scans. It is not recommended to use the axial imaging plane for size measurements in tortuous aortas as this may falsely overestimate the luminal diameter by making measurements off-axis; rather, a two-dimensional (2D) image can be reconstructed from a three-dimensional (3D) dataset allowing for a true on-axis cross section to obtain accurate measurements.
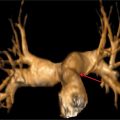
All true aneurysms of the aorta involve all 3 layers of the wall: intima, media, and adventitia. True aneurysms represent a focal dilatation of greater than 50% above the normal aortic diameter. There are 2 major classifications of true aneurysms—saccular and fusiform. Saccular aneurysms are focal distensions involving only part of the aortic circumference. Fusiform aneurysms are spindle-shaped with symmetrical dilatation involving the entire circumference of the aorta. Thoracic aortic aneurysms may involve more than 1 segment and are classified into different categories according to their anatomic region of involvement (Figure 8-5). Sixty percent of TAAs involve the aortic root and/or ascending aorta. However, pseudoaneurysms result from disruption of the arterial wall with extravasation of blood contained by periarterial connective tissue. Unlike true aneurysms, pseudoaneurysms do not involve all 3 layers of the vessel wall.
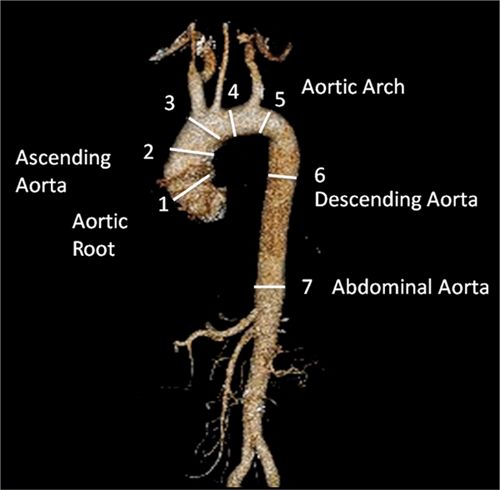
FIGURE 8-5 CT 3D reconstruction of normal anatomy of the thoracic and abdominal aorta. Thoracic aortic aneurysms may involve 1 or more segments of the thoracic aorta. Common measurements include (1) aortic root, (2) AA, (3) proximal aortic arch, (4) mid aortic arch, (5) distal aortic arch, (6) descending aorta, and (7) abdominal aorta.
DIFFERENTIAL DIAGNOSIS
Aortic Root Aneurysm
• Marfan syndrome
• Bicuspid aortic valve
• Turner syndrome
• Syphilis
Ascending Aortic Aneurysm
• Familial aortic aneurysm
• Marfan syndrome
• Bicuspid aortic valve
• Turner syndrome
• Atherosclerosis
• Syphilis
• Arteritis
Aortic Arch Aneurysm
• Syphilis
• Arteritis
Descending Aorta Aneurysm
• Atherosclerosis
• Trauma
MANAGEMENT
The goal of treatment is to reduce the risk of aneurysm rupture or dissection. One pivotal study identified a steep increase in rupture and dissection in aneurysms ≥ 6 cm in diameter with an annual rate of 7%.24
Beta-blocker therapy has been shown to be effective in slowing the rate of aortic dilatation, leading to fewer dissections, ruptures, and lower mortality in patients with Marfan syndrome with mild to moderate dilatation.25 Data is limited regarding the utility of beta-blocker therapy in non-Marfan patients. Nevertheless, given the overall relative safety of beta-blockers, it should be recommended to all patients with TAAs who tolerate therapy.6
Surgery is indicated for most ascending aortic aneurysms ≥ 5.5 cm.26 The threshold is raised to ≥ 6 cm in those individuals with multiple comorbidities. For individuals with Marfan syndrome and bicuspid aortic valve who are at elevated risk of rupture and dissection, surgical treatment is recommended for aneurysms ≥ 5 cm. When a bicuspid aortic valve is replaced, concomitant replacement of the AA is recommended if it is ≥ 4 cm. Surgery is advised for most descending aortic aneurysms ≥ 6 cm. In experienced centers, elective surgical repair of ascending and descending TAAs has a complication rate of 3% to 5% and 5% to 14%, respectively.6 More recently, a percutaneous transluminal approach for repair of descending aortic aneurysms has been developed.27 These endovascular stent grafts offer the advantage of being minimally invasive with less perioperative morbidity and mortality compared to surgery.
PATIENT EDUCATION
Aerobic exercise is generally well tolerated in patients with TAAs provided that systolic blood pressure (BP) remains < 180 mm Hg. Patients are advised to avoid strength training, including heavy lifting or straining, as these activities can rapidly increase intrathoracic pressure and BP. Patients should be educated on the typical red flags associated with TAA complications (see section on acute aortic syndromes).
FOLLOW-UP
One longitudinal study demonstrated that the rate of growth of all TAAs was 0.1 cm/y.28 Aneurysms that grow at a faster rate include descending aortic aneurysms, aneurysms associated with Marfan syndrome, aneurysms with larger diameters at diagnosis and dissected aneurysms. However, other studies have demonstrated that there is considerable variation in individual aneurysm growth rate.29 Hence, as outlined below, all aneurysms require serial imaging follow-up.
Patients with a history of bicuspid aortic valve should receive an initial TTE to assess the aortic root and the diameter of the AA.30 MRI or CT is recommended when morphology, the aortic root or AA cannot be accurately assessed by TTE.
Once a TAA is diagnosed it is recommended to follow-up with a subsequent imaging study in 6 months to identify rate of progression. Depending on the location of the aneurysm, this can be done using TTE, MRI, or CT. If there is no progression in diameter size at 6 months, annual imaging is recommended. We prefer to use MRI for serial studies to avoid repeated exposure to radiation and iodinated contrast. CT is used in patients with MRI contraindications or stent grafts. If possible, consistency with serial imaging studies using the same modality is advised at the same medical center for comparison to prior studies.
As many as 25% of patients with TAAs have concomitant AAAs (see section on abdominal aortic aneurysms). Therefore, it is recommended that all patients with a TAA undergo a baseline MRI or CT that includes the abdominal aorta.
ABDOMINAL AORTIC ANEURYSM
PATIENT STORY
A 72-year-old asymptomatic man with a history of obesity, hypertension, hyperlipidemia, and tobacco abuse underwent screening ultrasound for an AAA. On clinical examination, the patient had normal active bowel sounds; no abdominal bruit, palpable mass, or abdominal tenderness was noted. Abdominal ultrasound demonstrated evidence of an AAA measuring 4.8 cm; however, image quality was poor secondary to the patient’s body habitus. To better define the shape and extent of the aneurysm, an abdominal CTA was ordered which confirmed the ultrasound findings (Figure 8-6). Repeat abdominal imaging in 6 months was recommended to assess for any expansion.
EPIDEMIOLOGY
Abdominal aortic aneurysms are more common than TAAs. The incidence of AAA increases with age and approximately 5% of men who are more than 65 years old have an AAA.31 Men are 10 times more likely to have an AAA > 4.0 cm than women.32 However, AAAs in women are associated with a higher rate of rupture at a smaller mean diameter compared to men (5 vs 6 cm).33 Some AAAs are familial and tend to have greater complication rates at a younger age compared to sporadic AAA.34
Factors that increase the risk of rupture include larger AAAs, individuals with rapid expansion, those who continue to smoke, and those who have hypertension.33 There appears to be a steep rise in AAA rupture with diameters > 6.0 cm. The mean rate of AAA expansion is 0.4 cm/y.
ETIOLOGY AND PATHOPHYSIOLOGY
Smoking is the strongest risk factor for AAA. Other atherosclerotic risk factors such as hypertension and hyperlipidemia are also associated with AAA. The integrity of the abdominal aortic wall lies in its extracellular matrix, specifically elastin and collagen. The degradation of these proteins leads to weakening of the aortic wall and a predisposition for aneurysms. Historically, it was felt that atherosclerosis was the key mechanism in the pathophysiologic process of AAAs. However, the paradigm has shifted; it is now felt that AAAs occur from a complex interaction of genetics, hemodynamic, and immunologic factors as well as environmental triggers. Proteinases, such as plasminogen activators, matrix metalloproteinases, and cathepsins can be released from smooth muscle and inflammatory cells leading to degradation of elastin and collagen. Elevated levels of matrix metalloproteinases have been found in the walls of AAAs compared to controls.
DIAGNOSIS
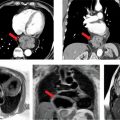
Various imaging modalities can be used to diagnose AAA. Abdominal ultrasonography (USG) is primarily used for screening purposes, especially in men > 65 years old with a history of smoking or those with a family history of AAA > 50 years of age.35 However, ultrasound is limited in its spatial resolution, determining the extent of the aneurysm and in evaluating the suprarenal aorta. As with measuring TAA, it is important to measure a true orthogonal aneurysm diameter when evaluating the abdominal aorta. When aortic angulation is greater than 25 degrees, axial imaging becomes unreliable.36 CT and MR angiography provide a comprehensive assessment of AAA size, shape and extent, relationship to adjacent structures, presence of mural thrombus and involvement of its branches (Figures 8-6 and 8-7). Novel techniques using CT and MRI measuring wall stress are promising in determining timing of aortic repair and may prove to be superior to maximum aortic diameter.37
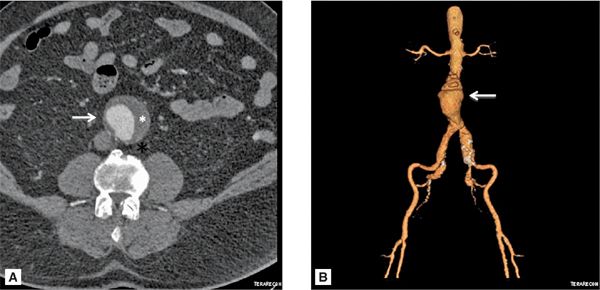
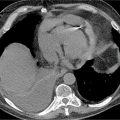
FIGURE 8-6 (A) Axial cross section through the aorta. (B) three-dimensional reconstruction of the same CT scan demonstrating the infrarenal AAA extending to the bilateral iliac arteries. White arrows indicate infrarenal AAA. * indicates thrombus in the aneurysm.
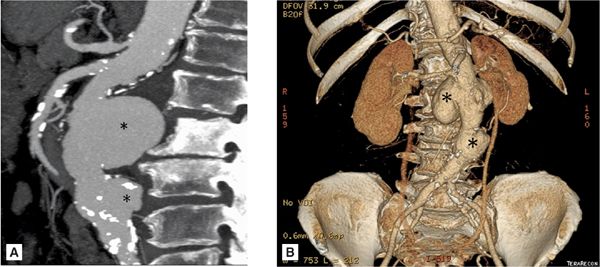
FIGURE 8-7 A 74-year-old man with endocarditis. (A) Contrast-enhanced CT scan demonstrating abdominal aortic aneurysms. (B) Three-dimensional reconstruction of the same CT demonstrating the multiple infrarenal abdominal aortic aneurysms considered to be mycotic in a patient with endocarditis. * indicates saccular aneurysm.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of symptoms associated with AAA includes:
• Irritable bowel syndrome
• Inflammatory bowel disease
• Renal colic
• Diverticulitis
• Ovarian torsion
• Splanchnic artery aneurysm/acute occlusion
• Low back pain
MANAGEMENT
The primary goal is to prevent AAA rupture. Abdominal aortic aneurysm rupture has a dismal 11% 30-day survival rate.33 Identifying patients prior to AAA complications is imperative, as elective AAA repair has less than a 6% mortality rate.
In most instances, surgery is not indicated in asymptomatic men with AAA < 5.5 cm.38,39 Women are at higher risk of aneurysm rupture at smaller diameters. Therefore, it is recommended that men with AAA ≥ 5.5 cm and women with AAA 4.5 to 5.0 cm should be considered for elective repair or when the diameter is greater than 4.5 cm and has increased at least 0.5 cm in the preceding 6 months.40 In low-risk patients, elective repair has a mortality rate of 2% in experienced centers. An alternative to open surgical repair is the percutaneous endovascular aneurysm repair (EVAR). Occasionally, a customized EVAR with fenestrations is designed with openings to maintain the patency of critical blood vessels. The EVAR serves as a bridge across the aneurysm excluding the aneurysm sac from blood flow (Figure 8-8). Eventually, the aneurysm sac clots and seals off. The rate of successful stent graft deployment is as high as 94%. An endoleak—a leak into the aneurysm sac—is one of the more common complications associated with EVAR placement. On CT, this can be diagnosed as persistent contrast flow into the sac from the aortic circulation (Figure 8-9). The endoleak can lead to an enlarging aneurysm size and potential aneurysm rupture. CTA has been a key contributor to the planning and success of this complex area of endovascular surgery.
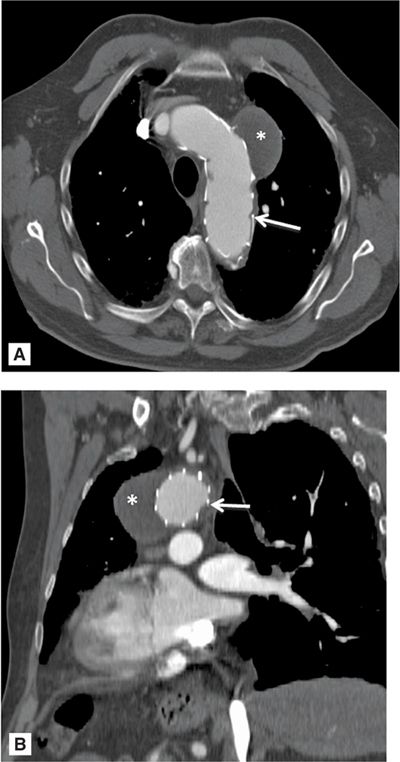
FIGURE 8-8 A 74-year-old man with a history of endovascular aneurysm repair (EVAR) of the aortic arch and descending aorta. Contrast-enhanced CT demonstrating a saccular aneurysm which has been sealed off and thrombosed (*). White arrow indicates eVar struts. A = axial orientation; B = oblique sagittal orientation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree