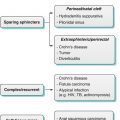
Algorithm 13.1 Algorithmic approach for evaluation of the appendix
Normal Appendix
A useful landmark for identifying the appendix at CT is the fatty ileocecal valve (Fig. 13.1). When trying to identify the appendix, it is helpful to trace the colon in a retrograde fashion until the fatty ileocecal valve is located. Once the ileocecal valve is identified, the base of cecum can be located, and the appendix can be identified arising from the cecal base. Pitfalls are to mistakenly identify the terminal ileum or a blood vessel as the appendix. Confirming that the structure thought to be the appendix is blind ending will avoid this pitfall.
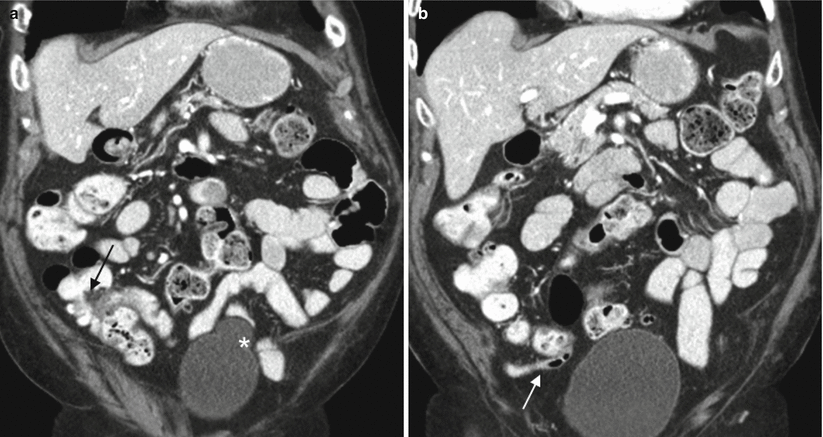

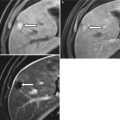
Fig. 13.1
Normal appendix at CT. Coronal contrast-enhanced CT images demonstrate a fatty ileocecal valve (a, arrow). The appendix (b, arrow) arises from the cecal base approximately 2–3 cm inferiorly. Incidentally noted bladder diverticulum (astrisk)
The appendix may terminate in a variety of locations including paracolic (62 %), pelvic (19 %), midline (9 %), and retrocecal (10 %) [17]. In pregnant women, the enlarging uterus may displace the cecum and appendix superiorly into the right upper quadrant, especially in the later stages of pregnancy [18].
A normal appendix will be thin walled, usually measure less than 10 mm in diameter, and will be surrounded by clean, black fat at CT and T2-weighted MR with fat saturation (Fig. 13.2). An awareness of normal appendiceal diameter and wall thickness is important as these are two key features assessed when evaluating for acute appendicitis. Appendiceal diameter can be quite variable at cross-sectional imaging as the appendix may be collapsed or distended with fluid and/or air [19]. Appendiceal outer-wall-to-outer-wall diameter in healthy, asymptomatic adults at CT ranges from 3 to 11 mm [17, 19, 20]. It is worth noting that greater than 40 % of normal individuals may have an appendiceal diameter of >6 mm at CT [19, 20] as a 6 mm “cutoff” is cited in the graded compression ultrasound literature as a useful cutoff for the diagnosis of acute appendicitis [21].
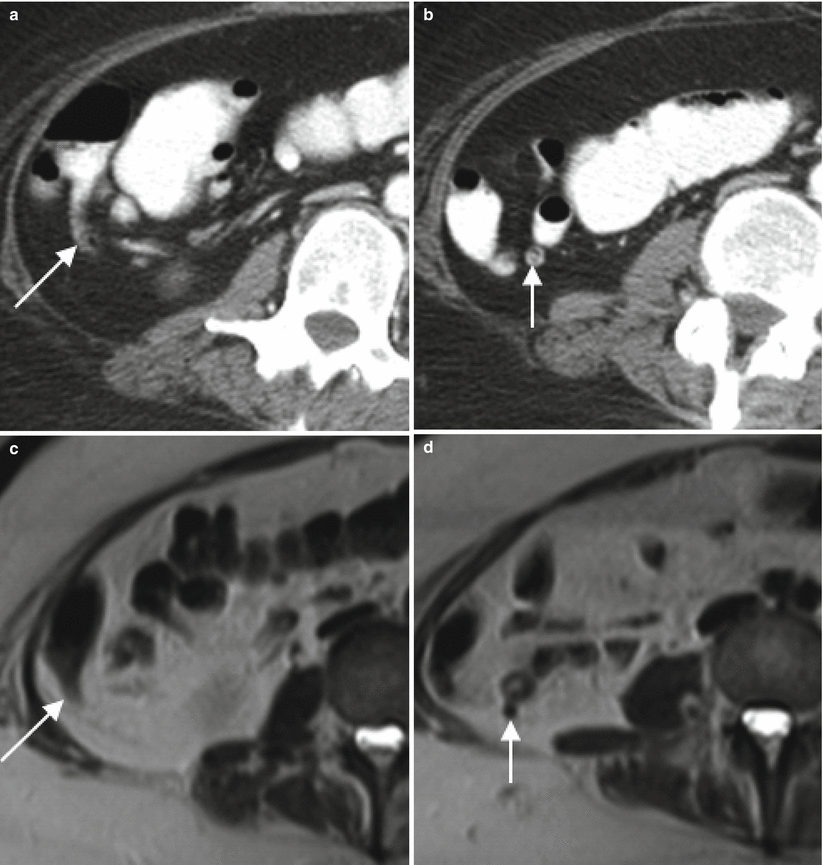
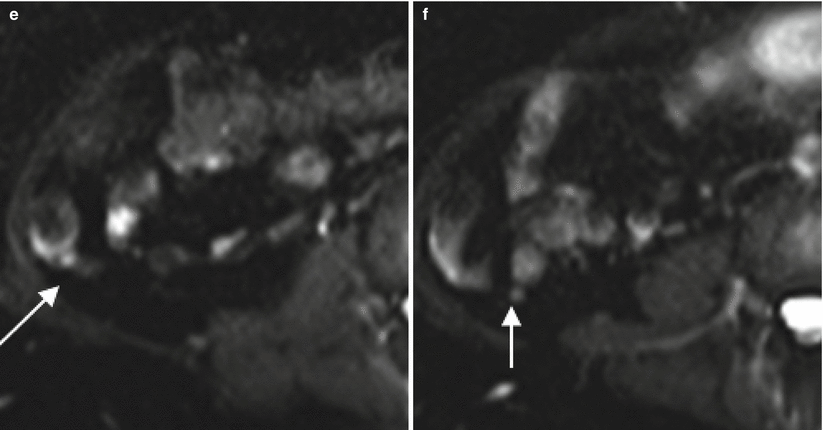


Fig. 13.2
The normal appendix at CT and MR. Axial contrast enhanced CT (a, b) and T2-weighted MR images without (c, d) and with (e, f) fat saturation demonstrate a normal appendix measuring 6 mm in diameter (arrows). Note also the lack of periappendiceal fat stranding
Appendiceal wall thickness also will vary depending on the degree of appendiceal distention. For example, the wall of the appendix is paper thin when the appendix is distended with air but may measure up to 3 mm in thickness when the appendix is collapsed. In other words, appendiceal wall thickness is normally <3 mm. Only 0.9 % of normal patients had an appendiceal wall thickness of ≥3 mm in one series [19].
Acute Appendicitis
As physical exam findings in patients with suspected acute appendicitis may be nonspecific, CT plays a valuable role in the workup of patients with possible acute appendicitis. Increasing rates of preoperative CT use in the United States have been shown to correlate with a decrease in rates of negative appendectomy (removal of a normal appendix in a patient preoperatively suspected to have acute appendicitis), especially in women of reproductive age [26] and in obese patients [27].
CT has a reported sensitivity of 90–100 %, specificity of 95–98 %, and accuracy of 94–98 % for the diagnosis of acute appendicitis [5, 28–30]. When interpreting a cross-sectional imaging study performed to evaluate for acute appendicitis, the first step is to identify the appendix. The next step is to evaluate for periappendiceal inflammation and assess the appendix for wall thickening and/or enlargement. A dilated, thick-walled appendix with periappendiceal fat stranding is diagnostic of acute appendicitis (Figs. 13.3, 13.4, and 13.5).
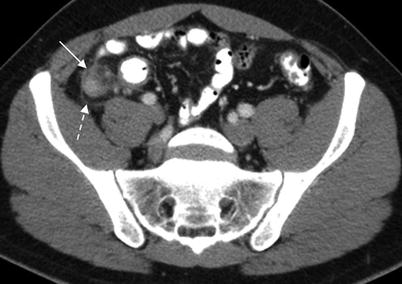
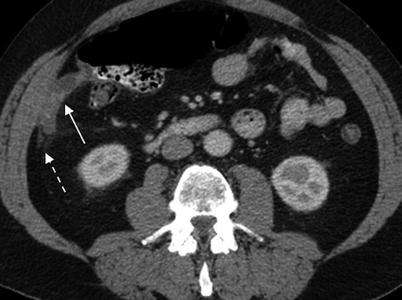
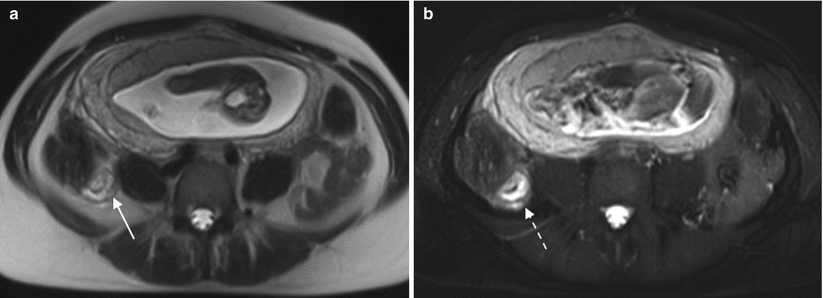

Fig. 13.3
Acute appendicitis. Axial contrast enhanced CT demonstrates dilated thick-walled appendix (11 mm diameter) (solid arrow) with periappendiceal inflammatory changes (dashed arrow)

Fig. 13.4
Acute appendicitis. Axial contrast enhanced CT demonstrates dilated thick-walled blind ended appendix (12 mm diameter) (solid arrow) with periappendiceal inflammatory changes (dashed arrow)

Fig. 13.5
Acute appendicitis in a pregnant patient. Axial T2-weighted MR images without (a) and with (b) fat saturation, demonstrate thick-walled appendix (solid arrow) with periappendiceal inflammatory changes (dashed arrow). Note that periappendiceal inflammation is much more apparent on the T2 with fat saturation image (b)
Appendiceal wall thickening is an extremely helpful finding for making the diagnosis of acute appendicitis [31, 32] and may be the only abnormal finding in a patient with early acute appendicitis. As noted above, appendiceal wall thickness will vary somewhat depending on the degree of appendiceal distention, but most normal appendiceal walls will measure <3 mm in thickness [19]. When interpreting cross-sectional imaging studies, it is recommended that the appendix be assessed in every study as so doing will build the reader’s internal memory of normal appendiceal wall thickness.
Appendiceal diameter also can be a helpful parameter, but should not be evaluated in isolation as a finding used to rule in or rule out acute appendicitis. Several numbers (>6 mm [33], >8 mm [34], ≥10 mm [17]) have been cited in the radiology literature as useful cutoffs for normal appendiceal diameter. But, as noted above, a normal appendix may measure >6 mm in diameter in >40 % of individuals [19, 20].
It is important to note that the 6 mm cutoff is based on ultrasound literature in which appendices were evaluated with graded compression [21]. As CT and MR are not performed with graded compression, a 6 mm cutoff should not be used for CT and MR. In fact, any strict diameter cutoff should be used with caution given the variability in normal appendiceal diameter noted above.
Periappendiceal inflammation appears as increased attenuation within periappendiceal fat at CT (Figs. 13.3, 13.4) or T2 bright signal within periappendiceal fat at T2 MR with fat saturation (Fig. 13.5). Periappendiceal fat stranding is highly suggestive of acute appendicitis if the appendix is enlarged [34]. However, periappendiceal inflammatory changes may also result from pathology centered outside of the appendix such as terminal ileitis in the setting of Crohn’s disease (Fig. 13.6), cecitis, or right ovarian pathology. Therefore, the appendix and surrounding structures must be evaluated carefully in the setting of periappendiceal fat stranding to accurately determine the etiology of fat stranding. Periappendiceal fat stranding also may be present in a small number of asymptomatic people at CT (3 % in one series) who are not ultimately found to have acute appendicitis [20]. Therefore, correlation should always be made with patient symptoms before a patient is diagnosed with acute appendicitis.
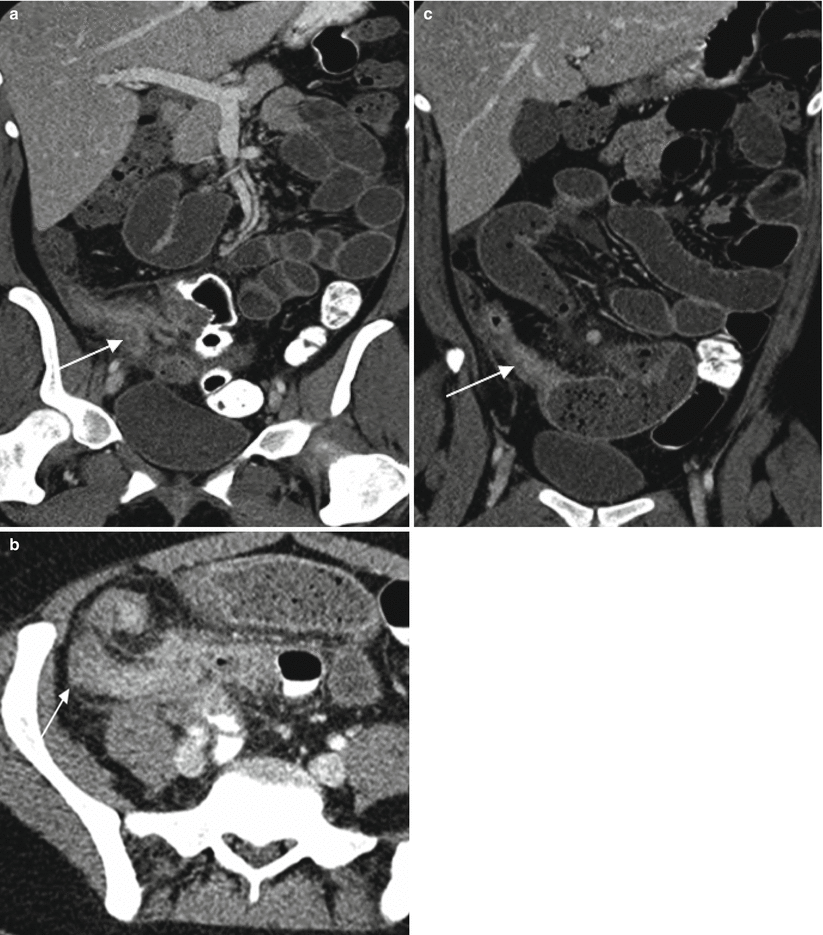

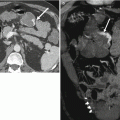
Fig. 13.6
Crohn’s disease of the appendix. Axial contrast-enhanced CT images demonstrate marked cecal wall thickening with surrounding inflammation (a, arrow) as well as wall thickening of the appendix with surrounding inflammation (b, arrow). Terminal ileum (c, arrow) is also thick walled with upstream loops of dilated small bowel. Crohn’s disease confirmed at histopathologic evaluation
If the appendix is not visualized and there are no inflammatory changes in the right lower quadrant, the incidence of acute appendicitis is low, 2 % in two series with positive cases having scant intra-abdominal fat [22, 23]. If the appendix is not visualized, there are no inflammatory changes in the right lower quadrant, and there is sufficient intraperitoneal fat in the right lower quadrant to assess for inflammatory changes, acute appendicitis can safely be excluded [23].
Once a determination of acute appendicitis has been made, the next step is to evaluate for evidence of perforation. Patients with perforated appendicitis may be prescribed antibiotics and undergo interval appendectomy at a later date after a “cooling-off” period rather than undergo surgery at initial presentation. Patients with perforated appendicitis and periappendiceal fluid collections may undergo CT-guided drainage of the fluid collections.
Findings indicating appendiceal perforation include periappendiceal fluid collections, extraluminal air, and an extraluminal appendicolith (Fig. 13.7). As noted above, CT is highly sensitive, specific, and accurate for the diagnosis of acute appendicitis. However, CT does not perform as well for the diagnosis of appendiceal perforation, and a number of highly specific but relatively insensitive CT findings of perforation have been described. For example, extraluminal air is diagnostic of appendiceal perforation in the setting of acute appendicitis, but is a relatively insensitive finding (35–36 % sensitivity in two series [35, 36]). Similarly, periappendiceal abscess and extraluminal appendicolith are also diagnostic of perforation in the setting of acute appendicitis but have low sensitivities of 34–36 % and 21 %, respectively [35, 36].
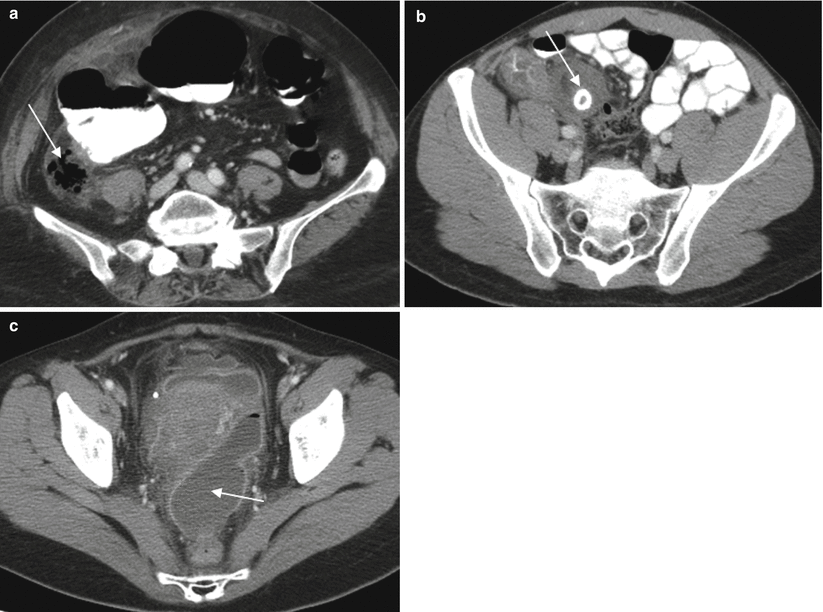

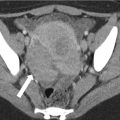
Fig. 13.7
Perforated appendicitis in three different patients. Axial contrast enhanced CT demonstrates extraluminal air (a, arrow), extraluminal appendicolith (b, arrow), and rim-enhancing pelvic fluid collection in keeping with abscess (c, arrow) are highly specific for perforated acute appendicitis. However, these findings have low sensitivity for perforated appendicitis (see text)
Appendiceal Neoplasms
Distinguishing appendiceal wall thickening due to appendicitis from wall thickening due to tumor can be challenging at CT [37]. However, as management of appendiceal tumors is, in general, right hemicolectomy rather than appendectomy, suggesting that appendiceal wall thickening may be due to tumor rather than inflammation or infection will alter management.
Carcinoid Tumors
Carcinoid tumors are the most common appendiceal tumor accounting for up to 80 % of appendiceal neoplasms [38]. The majority of these tumors occur in the distal third or tip of the appendix [37]. Appendiceal carcinoid tumors are found as incidental findings in appendectomy specimens in up to 1 % of patients [39, 40]. The majority of these usually incidentally found tumors are less than 1 cm in size, and appendectomy is considered to be curative [40]. Appendectomy is thought to be adequate treatment for carcinoid tumors less than 1.5 cm in size, whereas right hemicolectomy is recommended for carcinoid tumors >2.0 cm in size [41]. Carcinoid tumors are extremely difficult to identify prospectively at CT, likely due to their small size [37] but may appear as a well-circumscribed enhancing mass (Fig. 13.8), nodular thickening of the appendiceal tip or as more diffuse wall thickening [38].
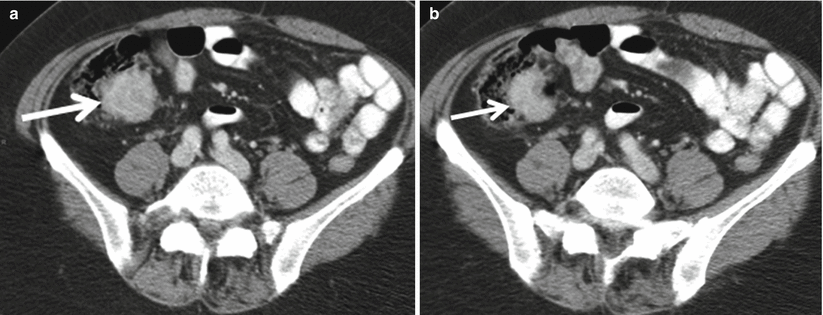

Fig. 13.8




Axial contrast-enhanced CT (a and b) demonstrates homogeneously enhancing mass lesion (arrow) involving the appendix, compatible with surgically proven carcinoid
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree