System
TIRADS 2
TIRADS 3
TIRADS 4a
TIRADS 4b
TIRADS 4c
TIRADS 5
Horvath 2009 [9]
Pattern
“Benign”
“Probably benign”
“Undetermined”
“Suspicious”
NA
“Consistent with malignancy”
Colloid type 1 nodule: anechoic avascular nodule with hyperechoic spots
Hashimoto’s pseudonodule: hyper-, iso-, or hypoechoic nodule with peripheral vascularization in Hashimoto’s
Simple neoplastic pattern: solid or mixed cystic/solid hyper-, iso-, or hypoechoic nodule with thin capsule
Malignant pattern A: hypoechoic nonencapsulated nodule with irregular shape and margins and penetrating vessels, with or without calcifications
Malignant pattern B: Iso- or hypoechoic nonencapsulated vascular nodule with multiple microcalcifications
Colloid type 2 nodule: nonencapsulated mixed vascular nodule with hyperechoic spots (spongiform)
DeQuervain pattern: hypoechoic lesion noncalcified with ill-defined margins
Malignant pattern C: Isoechoic mixed nonencapsulated vascular nodule with or without calcifications
Colloid type 3 nodule: nonencapsulated mixed nodule with isoechoic solid vascular portion with hyperechoic spots
Suspicious neoplastic pattern: hyper-, iso-, or hypoechoic hypervascularized encapsulated nodule with thick capsule containing coarse or microcalcifications
Estimated malignancy rate
0 %
<5 %
5–10 %
10–80 %
>80 %
Kwak 2011 [12]
Pattern
NA
“Probably benign”
“Low suspicion for malignancy”
“Intermediate suspicion for malignancy”
“Moderate concern but not classic for malignancy”
“Highly suggestive of malignancy”
Based upon the presence of five suspicious ultrasound features
Nodule with no suspicious features
Nodule with one suspicious feature
Nodule with two suspicious features
Nodule with three or four suspicious features
Nodule with five suspicious features
1. Solid composition
2. Hypo- or marked hypoechogenicity
3. Irregular or microlobulated margins
4. Microcalcifications
5. Taller-than-wide shape
Estimated malignancy rate
1.7 %
3.3 %
9.2 %
44–72 %
88 %
Pattern
“Benign”
“Very probably benign”
“Low suspicion of malignancy”
“High suspicion of malignancy”
NA
“Effectively certainly malignant”
High suspicion signs defined as
Simple or septated cyst, spongiform nodule, isolated macrocalcification
No high suspicion signs
No high suspicion signs
One or two high suspicion signs, no metastatic lymph nodes
Three to five high suspicion signs and/or the presence of metastatic lymph nodes
1. Taller-than-wide shape
Iso- or hyperechoic
Mildly hypoechoic
2. Irregular (speculated or microlobulated) borders
3. Microcalcifications
4. Markedly hypoechoic
5. High stiffness with elastography if available
Estimated malignancy rate
0 %
0.25 %
6 %
69 %
100 %
Park proposed thyroid imaging reporting based upon a logistic regression equation to estimate cancer risk that was derived from a database of 1694 nodules with ultrasound imaging and FNA results [11]. Each ultrasound exam was scored according to the absence or presence of 12 ultrasound features which resulted in a five-category scale: thyroid ultrasound (TUS ) 1–5. FNA was not recommended for TUS 1 and TUS 2. This system has not been widely adapted again because of the complexity inherent in categorizing multiple sonographic features and entering the score into a logarithmic equation to decide about FNA .
The goal of the second iteration of TIRADS , a system developed in Korea by Kwak et al. in 2011, was to develop a “practical” and less complex TIRADS stratifying malignancy risk and focused on FNA decision-making [12] (Table 14.1). These authors stressed that in parallel to BIRADS, differentiating category 3 from 4 was crucial because surveillance is recommended for the former and biopsy for the latter. In addition, they also introduced a 4c subcategory. The study included 1658 nodules larger than 1 cm that underwent FNA . In a multivariate analysis, five ultrasound features were associated with malignancy: solid composition, hypo- or marked hypoechogenicity, irregular or microlobulated margins, microcalcifications, and taller-than-wide shape. Using a derived regression equation, cancer risk exponentially increased with the number of suspicious ultrasound features present . With these findings, the authors proposed a new TIRADS classification system: TIRADS 3 no suspicious features, TIRADS 4a one suspicious feature, TIRADS 4b two suspicious features, TIRADS 4c three or four suspicious features, and TIRADS 5 five suspicious features. A main limitation of this classification system was each feature was assigned the same weight even though the authors noted that odds ratios for malignancy of certain features, such as irregular or microlobulated margins and microcalcifications, were higher than those of solid composition and hypoechogenicity. Furthermore, inherent in this type of checklist classification scheme is the assumption that each feature can occur independently of the other four and is therefore just as likely to be the only suspicious finding. For example, TIRADS 4a is defined by only one suspicious feature. This could certainly be solid composition, but it would be highly unlikely that microlobulated or irregular margins would be the one element defining TIRADS 4a nodule because these types of margins virtually always occur in solid hypoechoic or markedly hypoechoic nodules. Hence, a total of three characteristics are present, all of which are highly correlated, and these then define a TIRADS 4c nodule.
Subsequently, the Korean Society of Thyroid Radiology sought to modify the initial Kwak TIRADS system by assigning a different risk score to each suspicious ultrasound feature according to their odds ratios for cancer prediction derived from a multicenter retrospective study [13]. The total score associated with each nodule could then be used to predict malignancy risk. For example, the scores for hypo- versus marked hypoechogenicity were 2 and 6 points, respectively. No risk score was assigned to composition. However, what resulted from this new scheme was no longer a pattern recognition classification; rather, this scoring system reverted to identification of individual sonographic features and has not been widely adopted.
Also recognizing the differing probabilities of malignancy associated with the five ultrasound features, Russ and his colleagues proposed a third TIRADS classification scheme in 2013 [8], now referred to as the French system [14] (Table 14.1). Solid composition was removed from the list of high suspicion sonographic features. In addition, only marked, but not mild, hypoechogenicity was included in the list. High suspicion features were specifically defined as irregular shape, irregular borders, marked hypoechogenicity, microcalcifications, and, if available, high stiffness on elastography , but elastography was not considered prerequisite for this TIRADS classification. Mild hypoechogenicity in the absence of high suspicion features was categorized as TIRADS 4a. The presence of only one or two high suspicion features led to a TIRADS 4b category, but once three or more features were present, the nodule was classified as TIRADS 5. This system was validated in a prospective 2-year study of 4550 nodules (4.5 % prevalence of thyroid cancer) that underwent US FNA . In addition, the authors estimated a 34 % reduction in the number of FNAs if biopsy was not recommended for stable TIRADS 2 and 3 nodules [8].
Recognizing that the current TIRADS classifications have originated from individual institutions and none have been widely adapted in the United States, the American College of Radiology (ACR) has prioritized development of a TIRADS system, with the ultimate aim of applying it to risk stratification and triage of nodules for FNA or observation [15]. The initial step of the committee charged with this project was to develop a lexicon of defined sonographic features demonstrated to be consistently associated with either benignity or malignancy, which could be used to describe every thyroid lesion. The lexicon contains six ultrasound categories: composition, echogenicity, shape as taller than wide, size based upon maximal diameter, margins, and echogenic foci. Each category contains terms describing the varied appearances within that category, with discussion of the associated malignancy risk. The purpose of the lexicon is to develop a structured method for nodule evaluation and to decrease the variation of thyroid nodule reporting currently seen in the radiology practices in the United States. Although the authors recognized that nodule size is not generally correlated with cancer risk, the purpose of its inclusion is for the future development of ACR Guidelines for thyroid nodule management . The next step of this project, currently underway, includes statistical validation of the lexicon reporting system; therefore, this ACR TIRADS is still under development and not ready for clinical application.
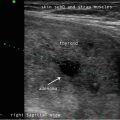
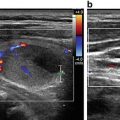
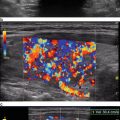
The current 2015 American Thyroid Association (ATA ) Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer sought to make pattern recognition more straightforward and reproducible for physicians who perform sonography on patients in their practices [16]. The ATA task force recognized the existence of three competing TIRADS systems, none of which has achieved universal adoption, as well as their perceived complexity by non-radiologists. The ATA Guidelines provide an “atlas” that offers examples of 15 sonographic nodule images and one image of sonographically suspicious lateral cervical lymph node. These 16 images are divided into five defined categories of sonographic patterns with associated cancer risks to triage nodules for FNA with recommendations for either surveillance or FNA with nodule size cutoffs (Fig. 14.1, Table 14.2). The patterns in each category were derived from a graded evidence-based literature review. The benign category includes the pure cyst, and FNA is not recommended, unless performed for therapeutic drainage. The very low suspicion patterns (cancer risk estimate <3 %) are spongiform nodules and mixed cystic solid nodules without high suspicion features defined as irregular (infiltrative, microlobulated) margins, microcalcifications, taller-than-wide shape, interrupted rim calcifications with small extrusive soft tissue component, evidence of extrathyroidal extension, or sonographically suspicious cervical lymph nodes. Low suspicion nodules (cancer risk estimate 5–10 %) include iso- or hyperechoic solid nodules or partially cystic nodules with eccentric solid areas without high suspicion features. Cancer risk increases to 10–20 % for intermediate suspicion nodules that are smoothly marginated, hypoechoic, and solid. The frequency of cancer is highest (>70–90 %) for the high suspicion category that includes solid hypoechoic nodules or solid hypoechoic component of a partially cystic nodule with any of the high suspicion features. Sonographic assessment of cervical lymph nodes is also prerequisite for all imaging as the presence of suspicious lymph nodules mandates FNA of the lymph node regardless of nodule pattern. Recommended FNA size cutoffs are smaller , 1 cm, for the patterns more likely to be associated with cancer and then increase as the likelihood of malignancy decreases, such that for the very low suspicion pattern observation may suffice.


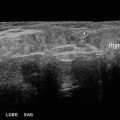
Fig. 14.1
American Thyroid Association nodule sonographic patterns and risk of malignancy
Table 14.2
ATA 2015 sonographic patterns, estimated risk of malignancy, and FNA guidance for thyroid nodules
Sonographic pattern | US features | Estimated malignancy risk (%) | FNA size cutoff (largest dimension) | TIRADS corresponding classification |
|---|---|---|---|---|
Benign | Purely cystic nodules (no solid component) | <1 | No biopsya | TIRADS 2 Horvath, Kwak, Russ |
Very low suspicion | Spongiform or partially cystic nodules without any of the sonographic features described in low, intermediate, or high suspicion patterns | <3 | Consider FNA at ≥2 cm | TIRADS 2 Horvath, Russ |
Observation without FNA is also a reasonable option
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|