Algorithm 5.1 Bile duct imaging patterns
Table 5.1
Biliary pathologies discussed in this chapter
Congenital | Infectious | Idiopathic | Autoimmune | Conditions due to stones | Benign neoplasm | Malignant neoplasm | Trauma and postop |
|---|---|---|---|---|---|---|---|
Bile duct cysts/choledochal cysts | Acute cholangitis | Primary sclerosing cholangitis | IgG4-related sclerosing cholangitis | Choledocholithiasis | Biliary cystadenoma | Cholangiocarcinoma | Bile leak |
Caroli disease | Recurrent pyogenic cholangitis | Mirizzi syndrome | Bile duct adenomas | Ampullary carcinoma | Biloma | ||
Biliary atresia | AIDS cholangiopathy | Papillitis | Biliary papillomatosis | Biliary cystadenocarcinoma | Benign stricture |
Normal Anatomy
Biliary anatomy (Fig. 5.1) parallels the portal venous supply of the liver. The right hepatic duct drains the entire right lobe of the liver. It is formed by the union of the anterior right hepatic duct, which drains the anterior segments V and VIII, and the posterior right hepatic duct, which drains the posterior segments VI and VII [1]. The anterior right hepatic duct lies vertically, whereas the posterior hepatic duct is more horizontal [2]. The left hepatic duct is formed by the union of ducts draining segments II and III and one or more ducts from segment IV [2]. Segment I normally drains to both the left and the right hepatic ducts. The bile ducts, together with branches of the hepatic artery and portal vein, form the portal triad.
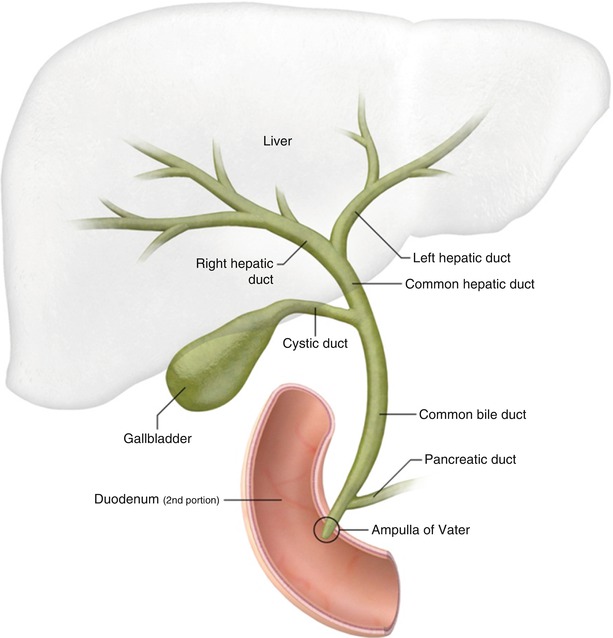

Fig. 5.1
Normal biliary anatomy
Normally, the right and left hepatic ducts converge to form the common hepatic duct at the porta hepatis. The common hepatic duct is joined by the cystic duct from the gallbladder to form the common bile duct (CBD). The CBD diameter is normally 6 mm or less (Fig. 5.2), but it may be larger in elderly patients or in those who have had their gallbladder removed. The CBD courses into the head of the pancreas and joins the main pancreatic duct to form the ampulla of Vater at the major duodenal papilla. The sphincter of Oddi is a circular band of smooth muscle that surrounds the distal end of the CBD and the main pancreatic duct at the level of the ampulla of Vater.
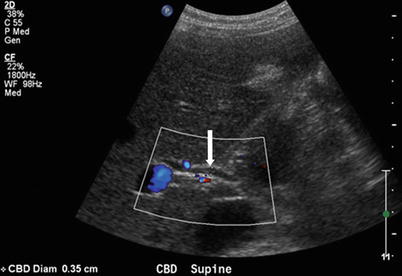

Fig. 5.2
Normal CBD. Color Doppler ultrasound image shows a normal caliber common duct (arrow) measuring less than 6 mm
Anatomic Variants
Variations in biliary anatomy are very common: the classic anatomy described above is present in only about 58 % of the population [3]. Magnetic resonance cholangiopancreatography (MRCP) is highly accurate in delineating bile duct anatomy for presurgical planning and is useful in determining whether variant anatomy exists, which may pose an increased risk of injury to the bile ducts during surgery [4].
Accessory bile ducts are aberrant ducts that drain individual liver segments. They usually arise from the right hepatic lobe but can arise from the left hepatic lobe or caudate lobe. Accessory ducts may drain into the common hepatic duct, the CBD, the cystic duct, or the gallbladder [5]. They can be too small to resolve by imaging but, if seen, should be included in the report if surgery is planned, since overlooking them at surgery may result in an insidious bile leak.
The most common anatomic variants of the biliary tree are depicted in Fig. 5.3. The course of the right posterior hepatic duct is the most variable. Normally, it joins with the right anterior hepatic duct to form the right hepatic duct. Alternatively, it can cross over to the left and drain into the left hepatic duct, termed a crossover anomaly (Fig. 5.4). This common biliary variant is present in 13–19 % of the population [6]. A common confluence of the right anterior hepatic duct, right posterior hepatic duct, and left hepatic duct is termed trifurcation and is present in 11 % of the population [3]. The right posterior hepatic duct occasionally drains directly into the common hepatic duct or, more rarely, into the cystic duct [7].
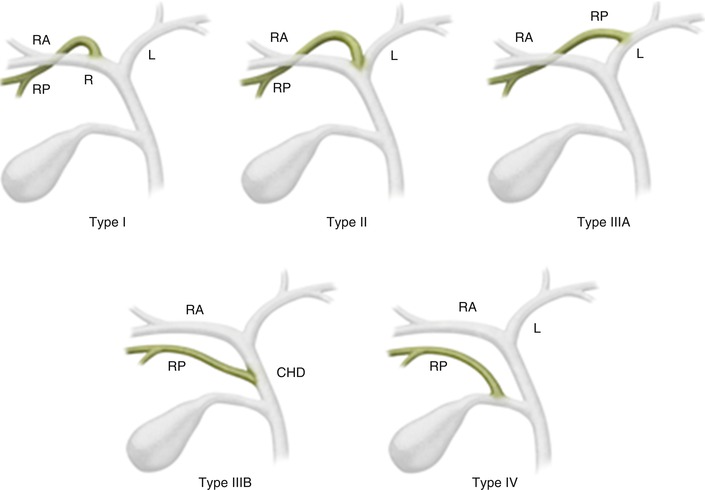
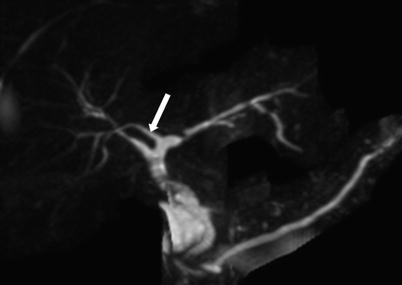

Fig. 5.3
Variant biliary anatomy. Type I: typical biliary anatomy. Type II: trifurcation. Type IIIA: right posterior hepatic duct draining into left hepatic duct. Type IIIB: right posterior hepatic duct draining into the common hepatic duct. Type IV: right posterior hepatic duct draining into the cystic duct

Fig. 5.4
Crossover anomaly. Coronal maximum intensity projection (MIP) image from MRCP demonstrates the right posterior hepatic duct (arrow) draining into the left hepatic duct, termed a crossover anomaly (Reprinted, with permission, from reference [7])
A crossover anomaly or trifurcation is important to recognize if a left hepatectomy is planned, since inadvertent ligation of the variant duct can lead to atrophy of segments VI/VII or V to VIII of the liver [7]. Ligation or resection of variant ducts, which may occur in laparoscopic cholecystectomy or in living-donor right-lobe transplantation, can lead to biliary leaks and strictures [7].
Imaging Techniques
In patients with suspected biliary disease, several imaging techniques can help determine the best management strategy.
Conventional radiographs are of little use in the diagnosis of biliary disease. Only 15–20 % of gallstones are visible on abdominal radiographs.
Ultrasonography (US) is frequently the initial study for evaluating the gallbladder and biliary system. A sonogram can usually be obtained rapidly, is cost-effective, does not use ionizing radiation, and is highly sensitive and specific at assessing gallstones and the presence of biliary dilatation. The sensitivity of US for detecting biliary dilatation increases with the degree of obstruction as reflected in the serum bilirubin level; US is approximately 95 % sensitive when the serum bilirubin is greater than 10 mg/dL. For patients with obstructive jaundice and suspected malignancy, the recommendation is to start with US, followed by magnetic resonance imaging (MRI)/MRCP or computed tomography (CT).
Endoscopic retrograde cholangiopancreatography (ERCP), an endoscopic technique for direct cholangiography, is primarily a therapeutic procedure for biliary disorders. With ERCP, the endoscopist can treat symptomatic biliary strictures or postoperative bile leaks with stents, can treat choledocholithiasis with sphincterotomy and stone removal, and can sample tissue for patients with suspected malignancy. Percutaneous transhepatic cholangiography can be used when ERCP is unsuccessful or when the patient has had prior anatomy-altering surgery, such as gastrojejunostomy, that would make ERCP difficult.
MRI and MRCP are excellent, noninvasive imaging techniques to evaluate for biliary disease. MRCP has advantages over ERCP in that it is noninvasive, safe, and cheaper, and it uses no radiation, requires no anesthesia, allows better visualization of bile ducts proximal to an obstruction, and, when combined with conventional MRI sequences, allows for detection of non-biliary disease [4].
MRCP is performed with heavily T2-weighted sequences, which substantially increase the signal of fluid-filled structures such as the bile ducts and gallbladder, resulting in a very high bile-to-background contrast. Fat saturation is generally used to suppress the background signal to allow for better delineation of the biliary system. Thick-slab sections provide an overview of the biliary system and resemble conventional cholangiograms. Thin-section multiplanar sequences and source images provide fine detail and are critical in evaluating for small intraluminal filling defects or strictures, which may be obscured by volume averaging on the thick-slab images [4, 8, 9]. MRCP demonstrates biliary dilatation, strictures, and intraductal stones with up to 95 % sensitivity.
MRI hepatobiliary contrast agents such as Gd-BOPTA and Gd-EOB-DTPA are partially excreted into bile. They can thus provide functional information about the excretion of bile, show biliary anatomical detail, and help detect bile leaks and assess the patency of biliary-enteric anastomoses.
CT, an excellent technique for evaluating complications of biliary and gallbladder disease, is particularly useful when the diagnosis is unclear or when alternative diagnoses need to be excluded. CT is sensitive for detecting biliary ductal dilatation, intrahepatic bile duct tumors, and the level of biliary obstruction. CT has the advantage of being less prone to artifacts compared with MRI. However, CT does not always visualize biliary stones and has limited sensitivity for determining intraductal tumor spread [10]. When evaluating for choledocholithiasis, thin collimation (2.5-mm) pre-contrast CT imaging with either no oral contrast or negative oral contrast (e.g., water) improves sensitivity for detecting stones.
Cholescintigraphy using Tc-99m-IDA (iminodiacetic acid) is useful for evaluating postoperative bile leaks.
Bile Duct Filling Defects
A stone is the most common filling defect within the biliary tree and may be primary, arising de novo within the biliary ducts, or secondary, arising from gallbladder stones passing into the CBD. Primary choledocholithiasis usually results from bile stasis and infection.
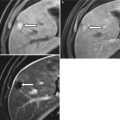
US has several limitations in the assessment of bile duct filling defects, such as its operator dependence, its reliance on a satisfactory acoustic window, and its difficulty in penetrating large patients or those with fatty livers. US has a low sensitivity of 25 % for detecting stones in the CBD (Fig. 5.5). Nevertheless, US is excellent for identifying biliary duct dilatation, and it has a high sensitivity for detecting gallstones. CT is superior to US for detecting CBD stones, especially when including indirect signs like ductal dilatation, but its sensitivity for direct depiction of CBD stones does not exceed 75 % [11]. CT is better at detecting calcified or gas-containing CBD stones (Fig. 5.6) but might miss stones that are isodense to bile (e.g., cholesterol stones) and can miss sludge or other small filling defects.
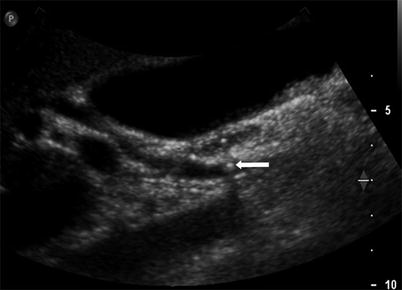
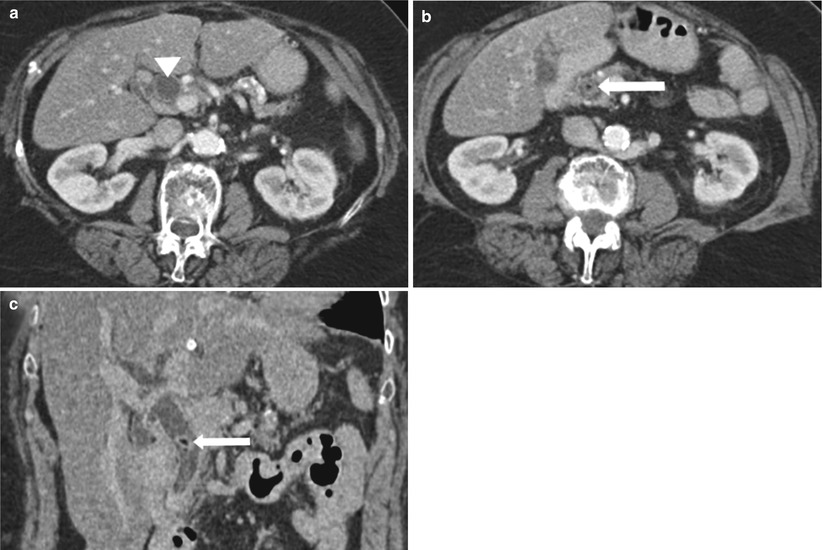

Fig. 5.5
Choledocholithiasis detected by ultrasound in a patient with a history of gallstone pancreatitis. Longitudinal US image shows a small round hyperechoic stone (arrow) with posterior shadowing within the CBD. There was no evidence of cholecystitis. The patient had several episodes of gallstone pancreatitis owing to the passage of gallstones

Fig. 5.6
Choledocholithiasis on CT. (a) and (b) Axial contrast-enhanced CT images show the dilated CBD (arrowhead) with a round intraductal filling defect representing the obstructing stone (arrow) in the distal CBD. (c) Coronal CT image shows the dilated CBD with an intraluminal stone (arrow)
Given the limitations of CT and US, MRCP has become increasingly favored to evaluate for biliary filling defects, especially in the preoperative setting. Filling defects can be clearly identified when surrounded by increased T2 signal bile. Stones are usually well circumscribed with sharp borders (Figs. 5.7 and 5.8), whereas intraductal tumors can show irregularity. Multiplanar evaluation helps exclude false positives. For example, pneumobilia may mimic a stone but will appear as a nondependent filling defect, whereas a stone will usually appear in a dependent position. Stones as small as 2 mm can be detected with MRCP; however, even larger stones may be missed if not surrounded by high-signal-intensity bile [12].
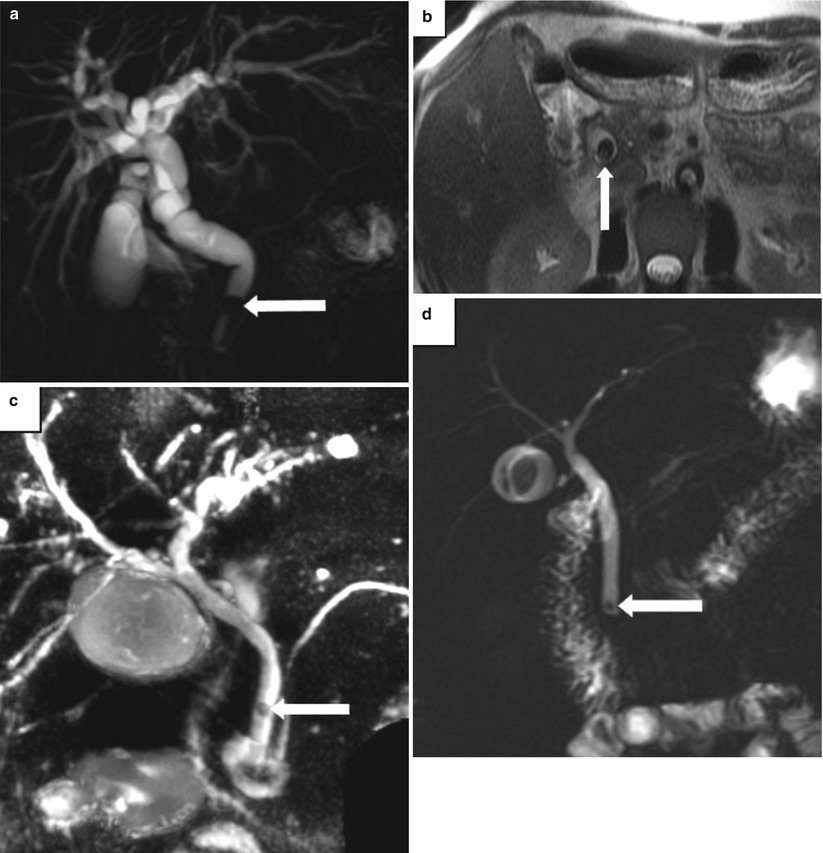
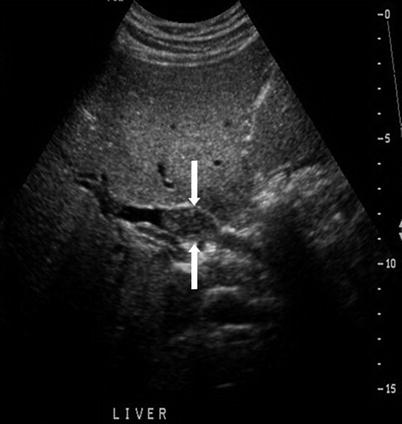

Fig. 5.7
Choledocholithiasis on MRCP and MRI. Examples of choledocholithiasis in three patients. (a) Coronal MIP image from MRCP and (b) axial T2-weighted MR image show an obstructing stone in the distal CBD (arrow) and diffuse intra- and extrahepatic biliary dilatation. (c) Coronal thick-slab MRCP image in a second patient shows a small, rounded filling defect representing a stone (arrow) in the mid-CBD. (d) Coronal MRCP image in a third patient shows a small stone in the distal CBD

Fig. 5.8
Intraductal-growing cholangiocarcinoma. Transverse US image shows a rounded, intraductal mass of intermediate echogenicity (arrows) representing an intraductal-growing cholangiocarcinoma. No acoustic shadowing is seen
A potential pitfall when evaluating MRCP images is artifact from arterial pulsatile compression of the extrahepatic bile duct appearing as a linear pseudo-obstruction on MIP images, usually from the right hepatic artery, which frequently passes immediately posterior to the common hepatic duct [13]. This pitfall can be avoided by careful interpretation of the coronal source images and the axial T2-weighted images to determine if a true obstruction exists. Susceptibility artifact from surgical clips or endovascular coils adjacent to the bile duct may result in signal loss, mimicking an obstructing lesion. Clips from laparoscopic cholecystectomy are usually titanium, which is nonmagnetic and does not cause signal loss [12]. Contraction of the sphincter of Oddi may also be a false positive for an impacted stone or stricture; however, this finding is transient, and a repeat MRCP when the sphincter is relaxed will show a normal configuration [12]. Evaluating the conventional MR images may also clarify the local anatomy and help exclude true pathology.
Bile duct adenomas are rare, occurring much less frequently than gallbladder adenomas. They may present with biliary obstruction or be found incidentally. On US images, they are intraluminal, nonshadowing masses, which may result in proximal biliary dilatation and may be difficult to distinguish from tumefactive sludge [14].
Biliary papillomatosis is a rare disease characterized by multiple papillary adenomas that arise in the intra- and extrahepatic biliary ducts. The adenomas are benign but have a high potential for malignant transformation and recurrence after treatment, leading to a poor prognosis. Clinically, patients present with signs and symptoms of biliary obstruction that may be complicated by acute cholangitis. Imaging reveals dilated bile ducts with multiple small hypoenhancing intraluminal masses. It may not be possible to distinguish biliary papillomatosis from malignant tumors such as polypoid cholangiocarcinoma (Fig. 5.8) and hepatocellular carcinoma with intraductal growth by imaging [14, 15].
Biliary Dilatation
In patients with clinically suspected bile duct obstruction, the role of imaging is to determine the presence and extent of biliary dilatation and, if owing to an obstructing lesion, define the level of and cause of the obstruction. Patients with biliary obstruction may present clinically with right upper abdominal pain, pruritus, jaundice, steatorrhea, dark urine, weight loss, nausea, and vomiting. Choledocholithiasis, the most common cause of biliary obstruction, is associated with acute intermittent right upper quadrant pain (biliary colic) and transient jaundice. Malignant bile duct obstruction, such as cholangiocarcinoma or carcinoma of the pancreatic head, may present with painless jaundice.
Bile duct obstruction results in elevated serum alkaline phosphatase (ALP) and gamma-glutamyltransferase (GGT) levels. Serum total and conjugated bilirubin levels are elevated in patients with more advanced obstruction and jaundice.
This section is organized into obstructive causes of biliary dilatation, including biliary stones, benign and malignant strictures, and biliary dilatation not owing to an obstruction See Alg. 5.2.
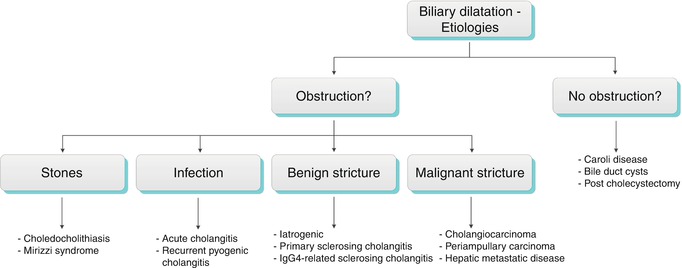

Algorithm 5.2 Etiologies of biliary dilatation
Table 5.2 lists several imaging features of biliary dilatation.
Table 5.2
Imaging features of biliary dilatation
“Shotgun” sign of dilated intrahepatic bile ducts on US. The dilated intrahepatic bile duct parallels the adjacent portal vein giving a double-barrel appearance. Color Doppler sonography distinguishes the bile duct from the portal vein (Fig. 5.9) |
Intrahepatic bile ducts proximal to the left and right hepatic ducts exceed 2 mm in diameter or are larger than 40 % of the diameter of the adjacent portal vein [16] |
Confluence of dilated bile ducts creates a stellate appearance (Fig. 5.9) |
CBD diameter >6 mm. In elderly patients or in those who have had their gallbladder removed, a CBD diameter up to 10 mm may be normal |
Several causes of biliary dilatation are depicted in (Fig. 5.10).
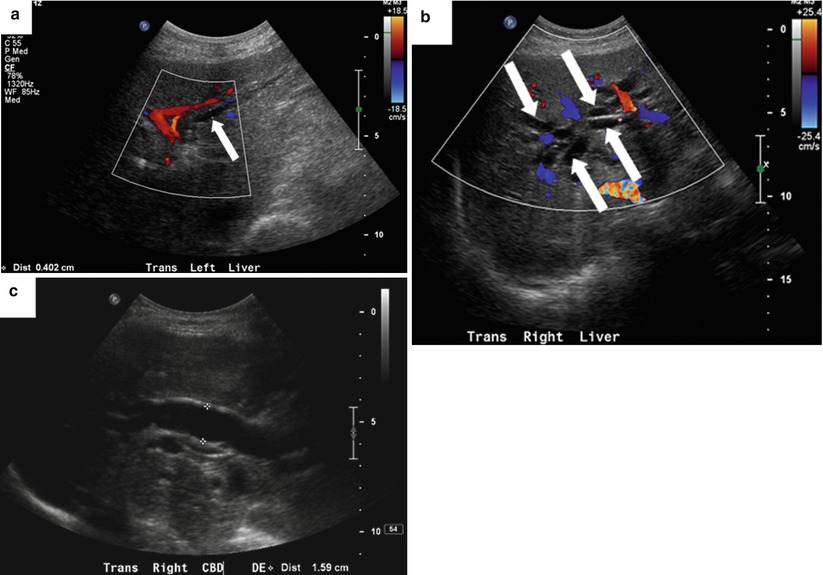
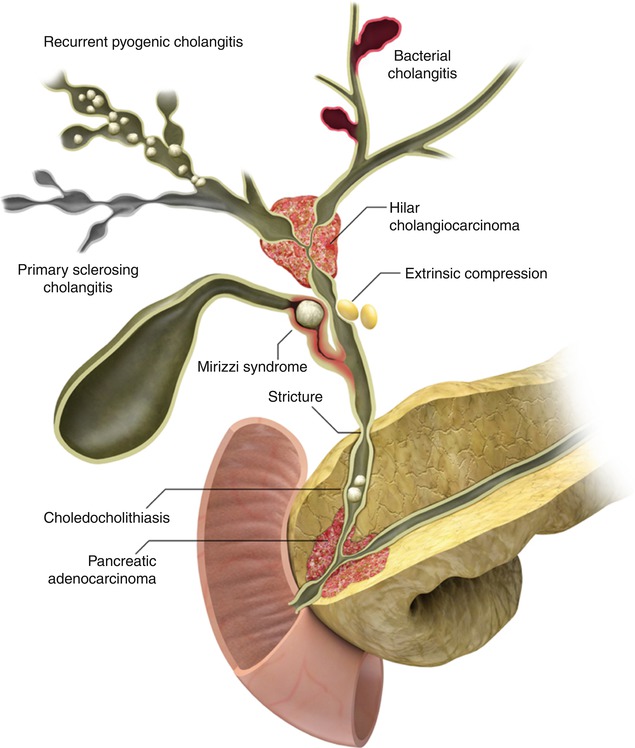

Fig. 5.9
Biliary dilatation—US signs. An 81-year-old woman with obstructive jaundice owing to ampullary adenocarcinoma. (a) Color Doppler US image shows a dilated intrahepatic bile duct (arrow) paralleling a portal vein branch. (b) Color Doppler US image shows a stellate appearance of dilated bile ducts (arrows) near the biliary confluence. (c) US image shows a dilated CBD measuring 16 mm

Fig. 5.10
Some causes of biliary dilatation
Obstructive Biliary Dilatation (Algorithms 5.3 and 5.4) [17]
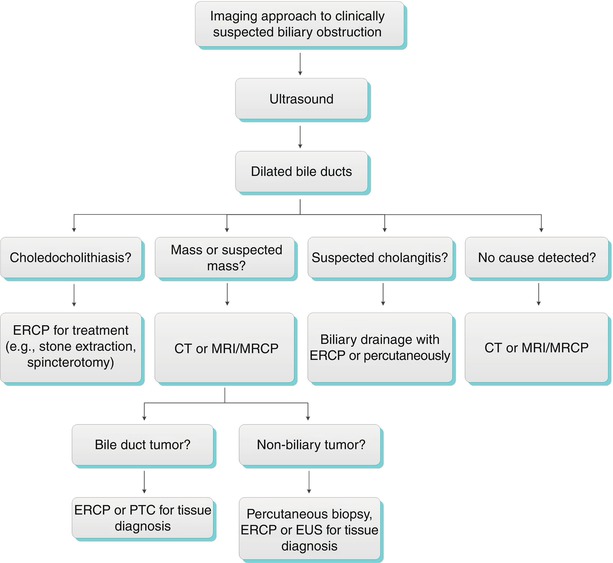
Algorithm 5.3 Imaging approach to clinically suspected biliary obstruction
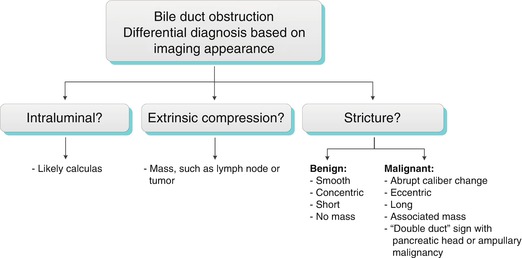
Algorithm 5.4 Bile duct obstruction: Differential diagnosis based on imaging appearance
Biliary Stones
Choledocholithiasis
Intraductal biliary stones can obstruct and cause extrahepatic and intrahepatic ductal dilatation upstream from the point of obstruction. Please see the preceding section on biliary filling defects for more information.
Choledocholithiasis is the most common cause of acute cholangitis and a common cause of acute pancreatitis.
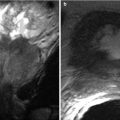
Occasionally, when a stone is impacted at the major duodenal papilla (Fig. 5.11) or has recently passed, the papilla may become inflamed, known as papillitis. Papillitis can be recognized on imaging when the papilla is enlarged (more than 5–10 mm in diameter), bulges into the duodenum (“bulging papilla”), and shows increased enhancement [18]. These findings may be indirect signs of an isoattenuating bile duct stone. A bulging papilla is not specific to choledocholithiasis and may be seen in cases of acute cholangitis, pancreatitis, periampullary diverticulum, choledochocele, intraductal papillary mucinous tumor, or periampullary tumors [18].
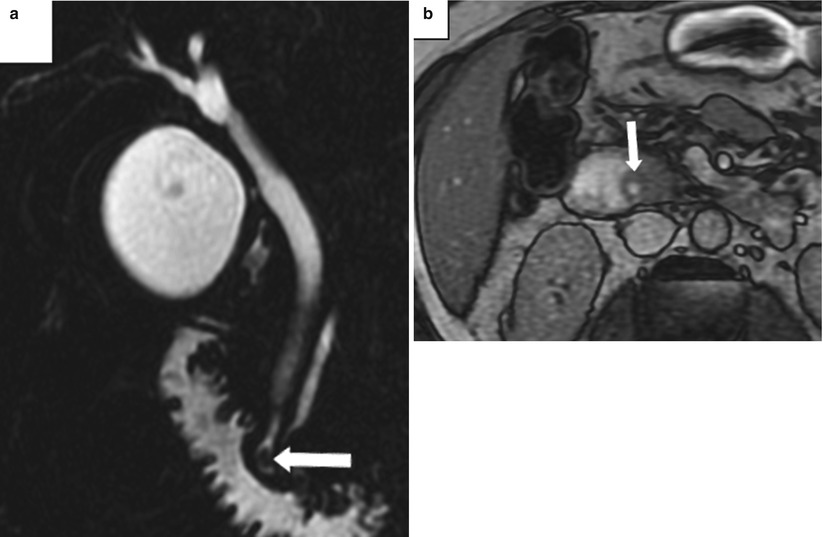

Fig. 5.11
Papillitis owing to an impacted stone at the ampulla. (a) Coronal thick-slab MRCP shows a small stone in the distal CBD impacted at the ampulla (arrow). (b) Opposed-phase T1-weighted MR images shows enlargement and bulging of the papilla (arrow) into the duodenal lumen (Reprinted, with permission, from reference [7])
Mirizzi Syndrome
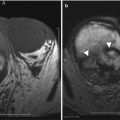
Mirizzi syndrome is a rare complication of gallstones in which an impacted stone in the cystic duct or gallbladder neck compresses the common hepatic duct, resulting in biliary dilatation (Figs. 5.12, 5.13, and 5.14). The common hepatic duct may become obstructed from the direct mass effect of the impacted stone (mechanical obstruction) or from secondary inflammation. Most patients present clinically with right upper quadrant pain, fever, and jaundice.
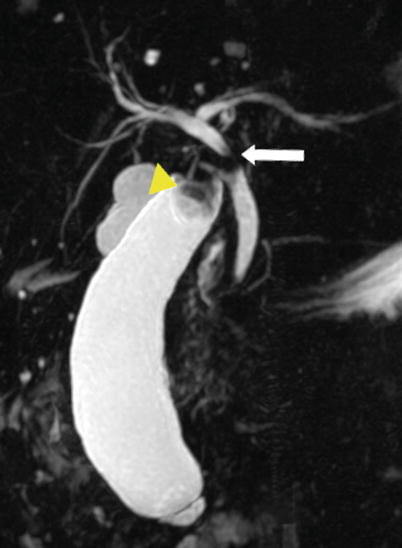
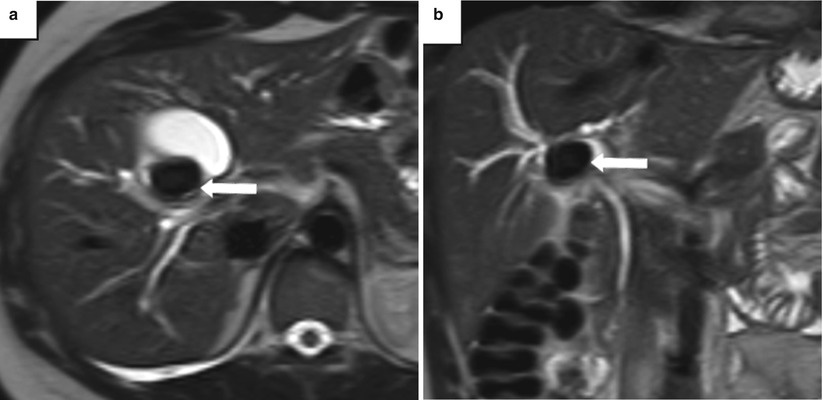
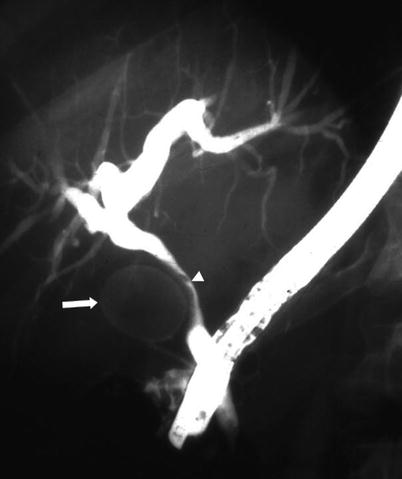

Fig. 5.12
Mirizzi syndrome—MRCP. Coronal thick-slab MIP image from MRCP shows a gallstone in the gallbladder neck (arrowhead) causing dilatation of the gallbladder and obstruction of the common hepatic duct (arrow) (Reprinted, with permission, from reference [7])

Fig. 5.13
Mirizzi syndrome—MRI. (a) Axial and (b) coronal T2-weighted MR images show an impacted gallstone (arrows) in the gallbladder neck causing mild dilatation of the intrahepatic bile ducts from mass effect on the common hepatic duct

Fig. 5.14
Mirizzi syndrome—ERCP. Fluoroscopic image from ERCP shows a large gallstone (arrow) compressing the common hepatic duct (arrowhead), resulting in diffuse intrahepatic biliary dilatation. On ERCP, Mirizzi syndrome is seen as smooth, focal, lateral scalloping of the common hepatic duct. There is nonvisualization of the gallbladder, as in this case, or opacification with a filling defect from the gallstone
Mirizzi syndrome is classified into four types based on the presence and size of a cholecystocholedochal fistula owing to erosion of the CBD wall by the impacted stone. Type I lesions have no fistula; types II–IV are subdivided based on the fistula’s size. The importance of identifying an underlying fistula is to guide therapeutic management—patients without a fistula can be treated by cholecystectomy alone, whereas patients with a fistula will require a cholecystectomy and fistula repair or a biliary-enteric anastomosis [7]. In rare instances, Mirizzi syndrome can occur following cholecystectomy if there is an impacted stone in the cystic duct remnant [19].
US and CT can suggest this diagnosis when a gallstone is seen within the gallbladder neck, the infundibulum, or the Hartmann pouch, in conjunction with dilatation of the bile duct proximal to the stone [20]. MRCP shows an impacted gallstone with associated dilatation of the biliary tree, with obstruction at the junction of the cystic and hepatic ducts. MRCP can also reveal associated gallbladder wall thickening and inflammation, especially with contrast-enhanced MRI [19].
Infection
Acute Cholangitis
Acute cholangitis, also known as ascending cholangitis, is a life-threatening disease caused by bacterial infection of bile. Choledocholithiasis, biliary interventions, and biliary stents are the most common predisposing factors. Other precipitating conditions include malignant obstruction of the CBD from cholangiocarcinoma, pancreatic cancer, cancer of the ampulla of Vater, metastasis, or the presence of a benign stricture (e.g., sclerosing cholangitis, iatrogenic). The infection is usually polymicrobial; gram-negative rods, especially Escherichia coli, are most commonly isolated from the bile, but gram positives and anaerobes are also frequently present. Patients typically present with fever (90 % of patients) and jaundice (60 % of patients). The classic “Charcot triad” of right upper quadrant pain, fever, and jaundice is seen in fewer than 70 % of patients [21]. If untreated, acute cholangitis carries a high mortality rate. Urgent biliary drainage, typically endoscopic or percutaneous, and broad-spectrum antibiotics are the cornerstones of treatment and often lead to rapid clinical improvement.
Contrast-enhanced MRI and CT, especially delayed images, show smooth, mildly thickened, enhancing bile duct walls (Fig. 5.15). MRCP demonstrates biliary obstruction in half of patients [7]. Liver abscess (Fig. 5.16) or portal vein thrombosis may be seen as a complication.
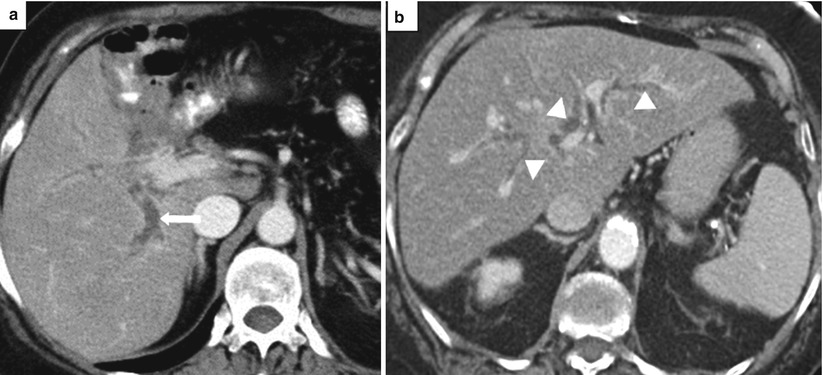
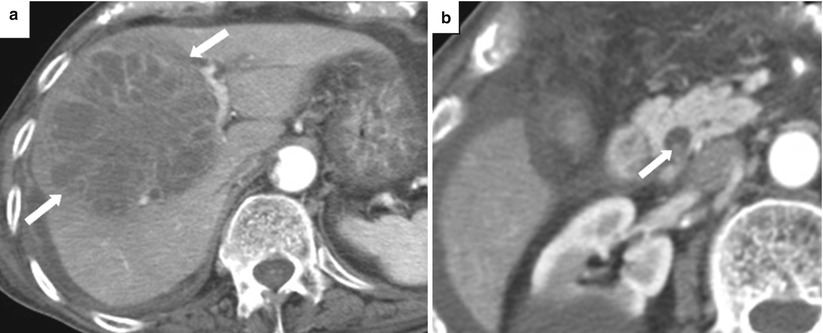

Fig. 5.15
Acute cholangitis. Axial contrast-enhanced CT images (a. b) show dilated intrahepatic bile ducts (arrowheads) with smooth, mildly thickened, enhancing walls (arrows)

Fig. 5.16
Liver abscess complicating acute cholangitis. (a) Axial contrast-enhanced CT image shows a large, complex, septated fluid collection in the liver owing to an intrahepatic abscess (arrows), complicating bacterial cholangitis. (b) A high-density filling defect seen eccentrically within the CBD represents a stone, a predisposing factor for cholangitis
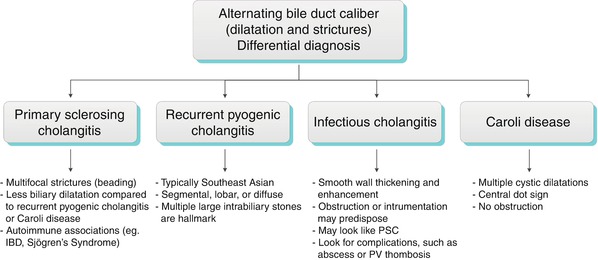
AIDS cholangiopathy is characterized by biliary obstruction resulting from multiple biliary strictures, most commonly as a result of inflammation from opportunistic infection with Cryptosporidium parvum, Cytomegalovirus (CMV), and Microsporidium. It is seen in patients with very low CD4 counts, usually well below 100 cells/mm3, and is not as common as it was before the advent of highly active antiretroviral therapy (HAART). Typical imaging findings include multiple, peripheral, long-segment biliary strictures and focal dilatation, which may appear similar to primary sclerosing cholangitis [7]. Involvement of the gallbladder may be seen.
Recurrent Pyogenic Cholangitis
Recurrent pyogenic cholangitis, also known as Oriental cholangitis, is characterized by multiple intrahepatic pigment stones resulting in stricturing and obstruction of the biliary tree leading to recurrent bouts of cholangitis (Fig. 5.17). The typical presentation is right upper quadrant pain, fever, and jaundice (Charcot triad) in a patient from Southeast Asia. Its underlying cause is unknown, but it has been associated with parasitic infections such as Ascaris lumbricoides and Clonorchis sinensis. Strictures and biliary stasis are thought to result from chronic inflammation of the bile ducts secondary to the infections. The intrahepatic biliary stones form proximal to the bile duct strictures.
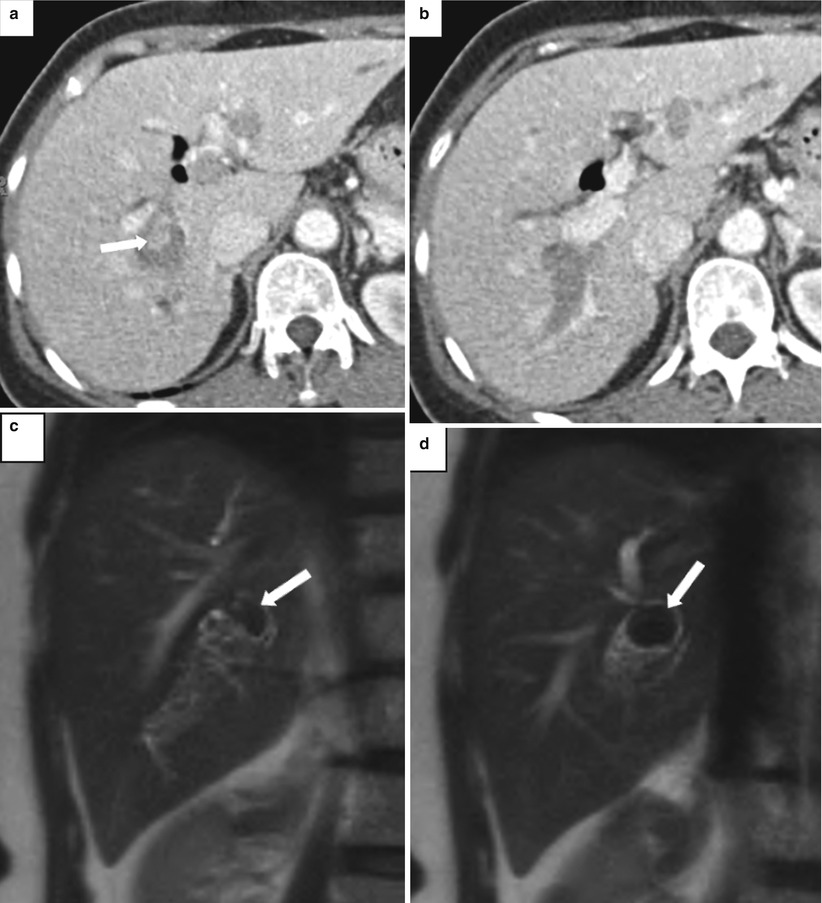
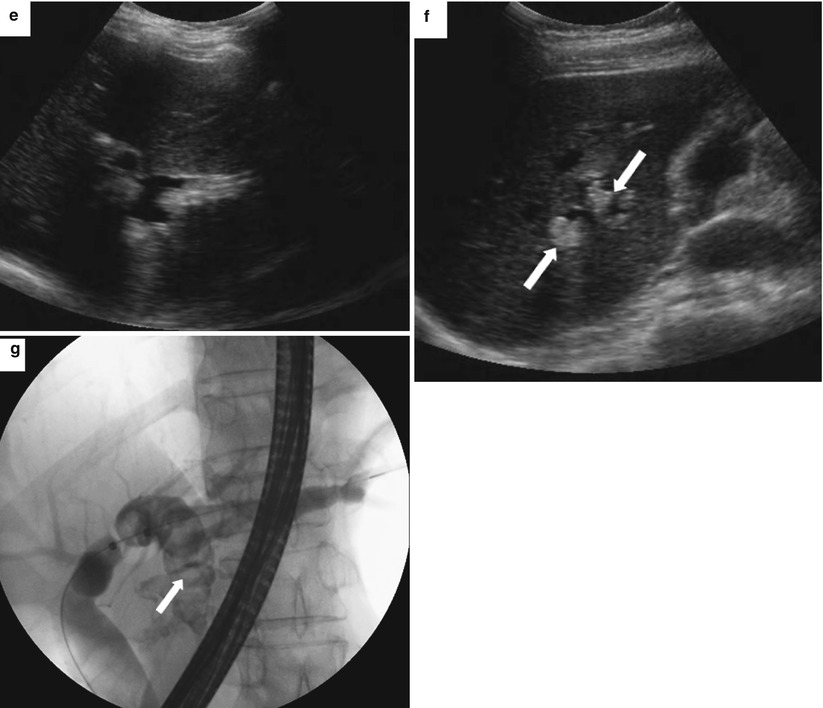


Fig. 5.17
Recurrent pyogenic cholangitis. A 53-year-old woman with right upper quadrant abdominal pain. (a, b) Axial contrast-enhanced CT images show pneumobilia, biliary dilatation, and a filling defect (arrow), representing a stone, in the bile ducts. (c, d) Coronal HASTE MR images show the large stone in the right hepatic duct (arrows). (e, f) US images show dilatation of the central intrahepatic bile ducts and multiple hyperechoic filling defects that represent stones (arrows). (g) Fluoroscopic spot image during ERCP shows the dilated left hepatic ducts with large lucent filling defects caused by the biliary stones (arrow)
US shows dilated intrahepatic ducts with stones and debris, and, uncommonly, hepatic abscesses may be detected. For uncertain reasons, the left hepatic duct, and specifically the left lateral segment duct, is more commonly affected; however, stones may be seen throughout the intrahepatic bile ducts as well as throughout the extrahepatic biliary tree.
MRI findings include marked intra- and extrahepatic ductal dilatation, with multiple focal strictures, and decreased arborization of the biliary tree. Multiple biliary stones of varying sizes, measuring up to several centimeters, are seen as filling defects with low signal on T2-weighted images and low-to-intermediate signal on T1-weighted images. On unenhanced CT, the stones may be equal to or greater than the liver density. Enhancement of the duct wall following the administration of contrast may be seen. MR and CT are also useful for detecting complications such as hepatic abscess (seen in 20 % of patients) and cholangiocarcinoma (seen in up to 5 % of patients) [7]. The liver parenchyma surrounding the affected bile ducts may be atrophied.
Biliary Strictures (Algorithms 5.5, 5.6, and 5.7)
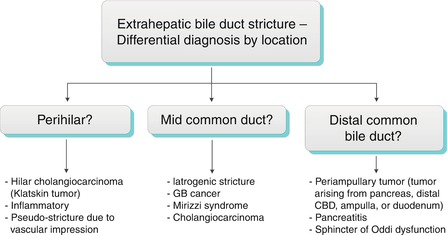
Generally, a smooth, concentric, short-segment stricture suggests a benign cause, which may be iatrogenic, inflammatory, ischemic, or from infection, whereas an abrupt, eccentric, long-segment stricture suggests malignant etiology [22]. These imaging features are not specific, however, and strictures with imaging features suggesting benignity may have a malignant etiology, and vice versa. Kim et al. found that contrast-enhanced MRI combined with MRCP was highly accurate in differentiating benign from malignant strictures, with significant factors for malignancy including wall hyperenhancement, length >12 mm, wall thickness >3 mm, an indistinct outer margin, luminal irregularity, and asymmetry of the strictured bile duct [23]. The anatomic location of bile duct strictures can help narrow the differential diagnosis as shown in Algorithm 5.4 “Extrahepatic bile duct stricture—differential diagnosis by location.”
Several tumor markers may also be useful in the work-up of malignant strictures. Serum carbohydrate antigen 19-9 (CA 19-9) levels may be elevated in cholangiocarcinoma and gallbladder cancer, whereas pancreatic cancer may have elevated carcinoembryonic antigen (CEA) levels, and hepatocellular cancer may have elevated alpha-fetoprotein (AFP) levels. Malignant biliary strictures with complete obstruction generally have much higher total serum bilirubin levels than do benign strictures, and a bilirubin level greater than 20 mg/dL is highly suggestive of malignant obstruction.
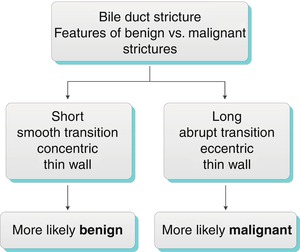





Algorithm 5.5 Bile duct stricture: Features of benign vs. malignant strictures

Algorithm 5.6 Extrahepatic bile duct stricture: Differential diagnosis by location

Algorithm 5.7 Alternating bile duct caliber: Differential diagnosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree