Sonography is a useful complement to physical examination, mammography, as well as magnetic resonance imaging (MRI) in the assessment of breast disease. The sonography examination provides a real-time tomographic display of the breast without ionizing radiation. In addition, the relative comfort of the examination and low cost make sonography an attractive choice for breast evaluation.
Mammography is the most common imaging modality used to evaluate the breast and remains the only widely used screening tool proven effective at reducing breast cancer mortality.
1,
2,
3,
4,
5 and
6 High-quality mammography is capable of detecting suspicious patterns of microcalcifications, which is typically the first imaging sign of a developing malignancy.
1,
2,
3,
4,
5,
6 and
7 Early cancer detection and treatment improves long-term survival by decreasing the incidence of lymph node involvement and metastasis to distant sites. Although digital mammography has improved pathology detection in certain breast types, mammography still has some diagnostic limitations. It cannot detect all breast masses. Lesions are more readily detected in a radiolucent, fatty breast and can be obscured in a radiopaque dense breast. Mammography alone does not determine whether a mass is cystic or solid because radiodensity can be similar. Localization of a mass is difficult if seen on only one radiographic view. Occasionally, superimposition of a focal area of normal breast tissues can create a pseudomass that may be difficult to differentiate from a real lesion on two-dimensional (2D) mammography. Digital breast tomosynthesis (DBT), or 3D mammography, allows breast tissues to be viewed “layer by layer,” which improves mass detection and reduces false-positive findings; however, not all patients may have access to this newer technology.
4,
5,
6 and
7 In addition, radiographic features of some breast malignancies are similar to those of benign masses so the level of diagnostic confidence is reduced.
For these reasons, adjunctive imaging tests are often recommended for breast evaluation in certain patients. When used in addition to mammography or physical examination, high-resolution sonography often improves diagnostic accuracy and assists in appropriate patient management.
CLINICAL ROLE OF BREAST SONOGRAPHY
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17 and
18Table 18-1 summarizes the indications and advantages of sonographic evaluation of the breast. Sonography’s proven ability to differentiate between cystic and solid masses allows palpable and radiographically indeterminate lesions to be characterized before invasive tests are considered. Dense fibroglandular tissue can hinder mass detection, especially on 2D mammography. Conversely, solid lesions are more evident on a sonogram when contrasted against hyperechoic fibroglandular tissue than in a less echogenic fat-replaced breast. Sonography serves as the primary nonionizing imaging modality used to evaluate symptomatic patients who are young, pregnant, or lactating, which are patient groups often associated with increased breast density that compromises radiographic evaluation.
Because sonography allows a tomographic display of the breast without painful compression, it is useful for examining patients with breast trauma, inflammatory changes, augmentation mammoplasty, or postirradiation changes. Sonography can also help evaluate the male breast for physiologic or pathologic changes. Mammography is often difficult to perform on these patients.
Following contrast-enhanced MRI evaluation, targeted “second-look” ultrasound (US) can assess an area of suspicious enhancement, a worrisome lesion, or an abnormal lymph node, especially when not visible by mammography. Careful correlation is needed owing to the differences in patient positioning between these modalities. If sonography can detect the MRI finding and image-guided intervention is recommended, then US guidance is typically preferred to MRI to biopsy a suspicious finding.
6,9Sonographic guidance is highly useful during the performance of interventional and therapeutic breast procedures. High-resolution, real-time sonography can assist needle placement during aspiration or biopsy procedures, affording direct visualization of the needle tip as it approaches and enters the mass. Evacuation of cystic lesions, drainage of abscess cavities, and sampling of suspicious solid masses and lymph nodes can be observed and confirmed. Clip marker placement to identify the site of mass after tissue sampling, or in conjunction with cancer therapy planning, can be performed using sonographic guidance.
Although sonography is useful in a number of clinical settings, there are limitations that should be recognized. The diagnostic quality of a sonographic breast examination is very operator and equipment dependent. As with other imaging modalities, differentiation between some benign and malignant solid lesions is less reliable when imaging patterns are similar. Even some normal structures and imaging artifacts (e.g., costal cartilage, Copper ligament shadowing) can be mistaken for pathology by an inexperienced breast imager. Newer high-frequency broadband transducers, harmonic imaging, spatial compounding, three-dimensional (3D) multiplanar imaging, and advanced processing techniques have increased sonography’s ability to assess lesion characteristics. Some manufacturers apply special processing filters to enhance the sonographic detection of microcalcifications.
19 However, the thin image planes and limitations in resolution restrict conventional sonography’s ability to assess the exact size, shape, and distribution patterns of microcalcifications within the breast that are readily detectable by mammography. Limitation in detecting suspicious calcifications is one reason why sonography is not approved as a “primary” breast cancer screening tool; however, sonography and contrast-enhanced MRI are most commonly used as supplemental screening tools to detect occult cancers in certain at-risk women and those with radiographically dense breasts when the efficacy of mammography is reduced.
1,
2,
3 and
4,20,
21 and
22Advancements in sonographic imaging such as automated whole-breast scanners, volumetric 3D and 4D imaging, image fusion technology, volume navigation, sonoelastography, contrast imaging, as well as development of computer-assisted detection (CAD) programs and lesion analysis software are some of the progressive changes to enhance sonography’s role in breast pathology recognition and diagnosis.
The breasts, or mammary glands, are paired, dome-shaped structures lying along the chest wall anterior to the level of the second to sixth ribs (
Fig. 18-1). As exocrine glands, the primary function of these modified sweat glands is to produce milk to nourish maternal offspring.
The breast tissue resides within thin layers of the superficial pectoral fascia. The anterior layer of this fascia travels just beneath the skin within the superficial fat, whereas the deep fascial layer lies near the fascia covering the pectoralis muscle. A layer of loose connective tissue between the deep layer of the superficial fascia and the pectoralis fascia is called the retromammary space. This potential space allows mobility of the breast over the chest wall.
The skin, nipple, and areola comprise the external surface of the breast. The outer epidermis and underlying dermis of the skin contain hair follicles, sebaceous, and sweat glands. The nipple is a round fibromuscular papilla projecting from the center of the breast that is encircled by the pigmented areola. Small bumps on the areola mark the sites of sebaceous glands (Montgomery glands), which secrete an oily substance to lessen drying and cracking of the nipple during breastfeeding.
The female breast is primarily composed of glandular, fatty, and fibrous connective tissues that vary in proportion based on the individual’s age and hormonal status. The glandular elements of the breast primarily function to produce and transport milk. The stromal elements consist of fat, fibrous connective tissues, and blood vessels, lymphatics, and nerves.
Between the skin and the chest wall, the breast is subdivided by fascial planes into three layers: the subcutaneous fat layer, the mammary (parenchymal) layer, and the retromammary fat layer. These breast layers are also referred to as the premammary, mammary, and retromammary layers or zones.
The parenchyma (fibroglandular tissue) contains the functional glandular elements of the breast and supporting connective tissues. Within this mammary layer are approximately 15 to 20 overlapping lobes arranged in a radial manner around the nipple. Each lobe contains 20 to 40 terminal ductolobular units (TDLUs), which are the functional units of the breast. A TDLU is composed of a lobule and its draining extralobular terminal duct. Each lobule contains an intralobular portion of the terminal duct that drains numerous, small terminal ductules (
Fig. 18-2). During late pregnancy, these ductules transform into tiny sac-like, milk-producing glands called
acini or
alveoli that involute after the lactation period ceases. Each lobe has a branching network of lactiferous ducts that transport milk from the TDLUs to the nipple. Smaller subsegmental and segmental ducts join to form a main lactiferous duct that becomes progressively larger. One major lactiferous duct empties each breast lobe and extends radially toward the nipple. Beneath the areola, the main duct focally enlarges at the ampulla or lactiferous sinus, which serves as a reservoir for milk during breastfeeding. Some major ducts from the lobes may join at the sinus level before exiting as excretory ducts through tiny openings at the summit of the nipple.
The ducts are lined by an inner epithelial cell layer and an underlying myoepithelial cell layer. The myoepithelial cells contract to help transfer milk out of the TDLUs during lactation. Breast ducts are surrounded by a basement membrane that separates ductal structures from adjacent tissues.
The TDLU is of histologic importance because most benign and malignant breast diseases arise from this
structure. The majority of the glandular tissue lies in the upper outer quadrant (UOQ) of the breast. The TDLUs are typically confined to the mammary layer; however, a TDLU may occasionally project between a Cooper ligament into the superficial breast or retromammary layer. A portion of mammary tissue may also extend into the axilla, forming the axillary tail of Spence.
The breast stroma helps provide support to the breast. Adipose tissue (fat) is found in the premammary (subcutaneous) and retromammary layers and fills out the spaces between the lobes and the lobules. Although the breast slides easily over the pectoralis major muscle, the gland itself is firmly attached to the skin by thin connective tissue bands known as Cooper ligaments. These suspensory ligaments extend radially through the breast from the deep pectoral fascia to the skin, enclosing fat lobules and providing structural support. Interlobular stromal tissue and Cooper ligaments make up the dense connective tissue. Hormonally responsive, loose intralobular stroma surrounds the smaller ducts of the lobule. Loose periductal fibrous tissue surrounds the larger ducts.
Posterior to the breast and pectoral fascia are muscles that separate the breast tissue from the chest wall. The pectoralis major muscle lies beneath the upper two-thirds of the breast. The smaller pectoralis minor muscle lies beneath the major muscle. The serratus anterior muscle extends under the lateral breast. The external oblique muscle is beneath a portion of the lower outer breast and the rectus abdominis abuts part of the lower inner breast.
The primary arterial blood supply to the breast is from the internal thoracic (mammary) artery and the lateral thoracic artery. The internal thoracic artery is a branch of the subclavian artery, whereas the lateral thoracic artery arises from the axillary artery. Additional blood supply includes the thoracoacromial and intercostal arteries. Arterial anastomoses occur beneath the areola.
Venous drainage is through superficial and deep networks. Venous anastomosis occurs in a circular pattern around the base of the areola (circulus venosus). Deep veins follow the path of the arteries to drain the breast, with most drainage heading to the axillary vein. There are connections between the intercostal veins and the vertebral plexus that are potential pathways for bone and nervous system metastasis.
Lymph vessels originate in the connective tissues near the lactiferous ducts and communicate with the subareolar plexus (Sappey plexus). Lymph drainage is from deep to superficial networks. Numerous lymphatics lie under the skin. Lymph vessels drain into lymph nodes and follow venous drainage pathways out of the breast. Small intramammary lymph nodes are found within the breast, especially in the UOQ and near the axilla. The vast majority of breast lymph drains into the ipsilateral axillary lymph node chain (˜75%). Lymphatic channels less often drain medially into the internal mammary (parasternal) nodes, which lie within the intercostal spaces alongside the sternum. Some lymph vessels drain to the opposite breast, toward the diaphragm, or to abdominal nodes. Axillary lymph nodes can be classified by anatomic location or by surgical level (
Table 18-2). For surgical and staging purposes, the axillary nodes are subdivided by level relative to the pectoralis minor muscle.
The nerves of the breast are located along the skin and within the glandular tissue. Innervation of the breast is primarily derived from branches of the lateral and anterior cutaneous branches of the second to sixth intercostal (thoracic) nerves. The fourth intercostal nerve serves the nipple. Superficial sensory nerves join the cervical, brachial, and intercostal nerves.
The anatomic components of the breast are easily demonstrated by high-resolution sonography. The appearance of the normal female breast varies widely from patient to patient and depends on the relative amounts of fat, connective, and glandular tissue in the scanning plane. Unlike 2D mammography, sonography allows sectional evaluation of the breast, one “slice” at a time, from the skin surface to the chest wall, allowing delineation of all breast layers or zones (
Fig. 18-3).
The skin layer is seen as two thin, reflective bands encasing a band of medium-level echoes representing the dermis. Scanning through an acoustic offset better delineates the skin. Normal skin thickness is usually 2 mm or less but slightly thicker near the areola and inframammary fold. The hyperechoic interface between the skin and subcutaneous fat should be intact. Changes in skin contour and thickness may indicate neoplastic, traumatic, postirradiation, or inflammatory changes at or below the skin surface.
The nipple displays a homogeneous texture of medium-level echoes. Connective tissue within the nipple and the irregular
contour of the nipple-areolar complex can cause acoustic shadowing. Probe compression and the use of ample scanning gel will flatten the nipple and reduce trapped air pockets to lessen shadowing. Transducer angulation beneath the nipple, the use of spatial compound imaging, or utilizing the two-handed peripheral scanning technique allows better evaluation of subareolar structures.
The premammary layer (subcutaneous fat layer) lies between the skin and the mammary layer and does not extend beneath the nipple. When equipment settings are properly set, the echogenicity of normal breast fat will be a midlevel gray shade. Blood vessels within this subcutaneous fat layer are easily compressed by transducer pressure. Cooper ligaments are best seen in the subcutaneous layer and imaged as thin, hyperechoic, curvilinear bands ascending from deep in the breast toward the skin and encasing the fat lobules. At times, streaks of acoustic shadowing are seen at oblique interfaces from these connective tissue ligaments, caused by refraction of the sound beam.
The mammary layer lies deep to the subcutaneous fat and contains the breast parenchyma and supporting tissues. This mammary zone is enclosed between the reflective interfaces of the anterior and posterior mammary fascia.
9,10 In the adult breast, the mammary layer has the greatest variation in echo pattern depending on the distribution of glandular, fibrous, and fatty tissues. Dense stromal fibrous tissue is very hyperechoic relative to fat, whereas glandular (periductal, lobular) tissue is nearly isoechoic to fat (
Fig. 18-4). Tubular fluid-filled ducts may be seen radiating from the nipple and into the breast core (
Fig. 18-5). Major lactiferous ducts are best imaged with radial scans. Generally, ducts measure 2 mm or less but are often larger at the lactiferous sinus and during lactation.
The retromammary layer lies between the posterior mammary fascia and the pectoralis major muscle and contains small fat lobules and some suspensory ligaments. This retromammary fat layer appears thinner on a sonogram than the subcutaneous fat layer.
At the chest wall, the pectoralis major muscle lies immediately posterior to most of the breast. The pectoralis minor muscle lies beneath the major muscle in a more superolateral location. These muscles appear as striated hyperechoic and hypoechoic linear bands of echoes that run parallel to the chest wall. Recognition of the pectoral major muscle is important to confirm ample US penetration of the breast and to help determine whether a deep lying cancer is invading through the pectoral fascia.
Deep to the pectoral muscles are the ribs and intercostal muscle of the thoracic cage. Laterally, the bony portion of the ribs attenuates the sound beam, resulting in acoustic shadowing. Medially, the costal cartilage of the ribs appears as oval structures containing low-level echoes seen best on sagittal scans (
Fig. 18-6). Care must be taken not to mistake the cartilaginous portion of the ribs for a breast mass. Rotating the transducer in orthogonal scan planes will confirm
the cartilage is not a mass. The hyperechogenic interface beneath the chest wall demarcates total sound reflection from the lung.
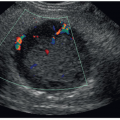
Normal lymph nodes can be imaged by sonography, especially in the axilla. Normal intramammary nodes are less often detected. The size of a normal intramammary node is typically less than 1 cm, but axillary nodes can be larger. Normal nodes are oval or reniform in shape, circumscribed, and display a symmetrically thin hypoechoic outer cortex and a hyperechoic fatty hilum (
Fig. 18-7). Color Doppler identifies a single feeding artery and draining vein at the hilum. Axillary nodes that contain mostly fat and have a very thin cortical rim may be difficult to delineate from adjacent structures. Enlarged lymph nodes may indicate inflammatory or neoplastic change.
VARIATIONS IN NORMAL PATTERNS
The proportionate amount of stromal and glandular tissues in the female breast depends on the patient’s age, parity, and whether she is premenopausal or postmenopausal, pregnant or lactating, or obese. The prepubertal breast is small and fatty. At puberty, estrogen and progesterone stimulate breast development by promoting duct growth and lobular development. The adolescent breast becomes increasingly glandular, whereas the amount of surrounding fat diminishes.
The adult breast has the greatest range of appearances. Characteristically, the young, nulliparous female breast is densely glandular, with little internal or surrounding fat. Sonographically, the glandular tissue appears isoechoic to mildly hyperechoic compared with fat. Over time, more hyperechoic fibrous stroma is apparent within the mammary zone that can partially attenuate the sound beam. With increasing age and number of pregnancies, fatty replacement of the parenchyma begins to occur. Isolated, hypoechoic fat lobules in the mammary layer may appear “mass like” compared with the more echogenic surrounding fibroglandular
tissue; however, fat lobules are very compressible and often merge into other fat lobules on the orthogonal scan plane.
In the breast of the pregnant or lactating woman, glandular elements proliferate within the mammary layer, compressing the surrounding fat layers. Acini within the breast lobules develop and begin milk secretion in response to hormones that include placental lactogen, chorionic gonadotropin, and prolactin. The overall texture of this glandular parenchyma can become weakly echogenic compared with denser connective tissues within this layer. Duct dilatation may be marked during late pregnancy and lactation.
The postmenopausal breast may show complete or near-complete fatty replacement as the lobules and ducts atrophy as hormonal influences decline. Cooper ligaments are easily seen encasing the fat lobules. However, older, nulliparous women or those on hormone replacement therapy (HRT) may retain considerable amounts of glandular tissue.
In both genders, breast development begins in utero along bilateral ectodermal milk lines that extend from the axilla to the inguinal region. Although there are several points along the milk lines for breast tissue to develop, one paired set of breasts grow from mammary ridges located in the thoracic region. Rudimentary ducts arising from epithelial breast buds are present in both females and males at the time of birth. In the neonate, there may be some transient breast enlargement and milky nipple discharge because of residual circulating maternal hormones.
Developmental anomalies of the breast are uncommon. Some are associated with abnormal endocrine gland development or hormonal dysfunction and may not become apparent until puberty. Congenital nipple inversion is a normal variant. An accessory nipple (polythelia) is the most common congenital breast anomaly. A completely formed accessory breast (polymastia) is rare. These supernumerary anomalies can occur anywhere along the milk line. Accessory breast tissue in the axilla (without a nipple) is occasionally present. Underdevelopment (hypoplasia) or excessive growth (hypertrophy) of the breast tissue may affect one or both breasts. Athelia indicates congenital absence of the breast nipple. Amastia refers to absent development of the nipple, areola, and breast tissue. Poland syndrome is associated with underdevelopment or absence of the breast, nipple, and chest muscles. Amazia refers to absence of breast tissue development although the nipple is present. This acquired condition may be secondary to excessive chest wall radiation or inadvertent excision of premature breast tissue development in a child.
Before the age of 8 years, growth of one breast occasionally occurs before the other. By puberty, however, both breasts are usually comparable in size and development.
24 This variant, referred to as unilateral early ripening or unilateral premature thelarche, should not be mistaken for pathology and does not necessitate excision. Early developing glandular tissue images as a small, mildly hypoechoic region under the nipple corresponding to the palpable region of subareolar nodularity.
If development of both breasts occurs before 8 years of age, precocious puberty is suspected. Causes are varied. Sonographic signs of precocious puberty may include ovarian enlargement with prominent follicular cysts, the presence of a hormone-secreting ovarian tumor (e.g., granulosa-theca cell tumor), a large-for-age uterus, or an adrenal tumor.
BREAST SONOGRAPHIC EXAMINATION AND INSTRUMENTATION
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17 and
18Before scanning, the indication for the examination, as well as any pertinent prior mammograms, breast sonograms, and/or other correlative imaging tests, is reviewed. The clinical history and the location of palpable masses, biopsy scars, and other changes of the skin, nipple, and breast contour are documented. Correlation of clinical and relevant imaging tests with the sonographic findings is imperative so the interpreting physician can more accurately assign a risk classification, provide a differential diagnosis, and make recommendations for patient management.
The American College of Radiology (ACR) publishes practice parameters and minimum equipment requirements for the performance of breast sonography.
16,
17 and
18 Proper transducer selection, equipment setup, patient positioning, and scanning technique are essential to produce high-quality images.
Transducer Requirements
Based on 2016 ACR practice parameters, breast sonography should be performed utilizing a high-resolution, real-time, linear-array, broad-bandwidth transducer operating at a center frequency of at least 12 MHz and preferably higher.
4,16 Ultra-broadband transducers are now available that span even wider frequencies ranges, up to 15 to 18 MHz at the higher end (e.g., L17-5; eL18-4). High-frequency, broadbandwidth transducers provide better spatial and contrast resolution. Higher frequencies enhance image resolution and optimize detail of more superficial structures, whereas lower frequencies allow better penetration of attenuative tissues and deep lying structures. During the examination, the highest frequency capable of adequate sound penetration of the region of interest should be used.
Equipment and Grayscale Settings
Equipment settings must be optimized to display an image that clearly demonstrates all breast tissue from the skin line to the chest wall or for the selected region of interest. Handheld transducer footprints of 35 to 50 mm enable effective survey scanning of the breast. Important system controls include output power, time gain compensation (TGC), overall gain, dynamic range, as well as optimization of focal zones, image size, and image depth. In addition, image optimization is augmented by using harmonics, spatial compounding, speckle reduction, and speed-of-sound correction features, as applicable.
Appropriate grayscale setup is important for breast sonography. The gain settings, dynamic range, and processing curves should be set so that normal breast fat displays a medium-level gray shade. Using accepted ACR lexicon, the echogenicity of other breast structures and masses are described as being hypoechoic, isoechoic, or hyperechoic compared with normal (subcutaneous) fat.
4,6 Correct TGC settings will allow normal breast fat to be displayed with
the same echogenicity within the premammary, mammary, and retromammary breast layers.
Proper sound beam focusing reduces volume-averaging artifacts and enhances detail of the area of interest. During survey of the breast, the number of focal zones should be increased to span depth of the mammary zone. When evaluating a mass, the focal zone should be placed at, or slightly below, the level of the mass to enhance resolution and posterior acoustic effects. Matrix-array transducers allow elevation plane focusing to further enhance near-field imaging.
Patient Positioning
Patient positioning during breast sonography can vary depending on breast size, mass location, or as needed for sonomammographic correlation. In general, the patient should be positioned in a manner that minimizes the thickness of the portion of the breast being examined. The patient is typically placed in a “supine-oblique” or “contralateral-oblique” position. This is accomplished by rolling the supine patient approximately 30 to 45 degrees, allowing placement of a wedge support to elevate the side of the body to be examined. The adjacent arm is extended near the head, which helps to stabilize the breast and provides access to the axillary region. The breast should appear flattened with the nipple centered. The reduction in breast thickness allows sound penetration by higher frequencies and orients major tissue planes more parallel to the face of the transducer optimizing sound beam reflection. This patient positioning can be used to scan the entire breast but is particularly useful when examining the outer breast. Steeper obliquity may be needed to scan large breasts or very lateral lesions. A straight supine position is often preferred when examining medial lesions.
Sometimes, a patient can only feel a mass while in a specific position. Scanning the patient in that position allows correlation of clinical and sonographic findings. For more accurate sonomammographic correlation of a breast mass, it may be helpful to scan certain patients in positions that simulate the mammographic views.
Scan Procedure and Technique
Based on site protocol and indication, either a targeted examination or a whole-breast sonographic examination is performed utilizing a handheld, high-resolution transducer. Targeted examinations are more common and limited to the quadrant or region of clinical concern, such as for a palpable mass, or to further characterize a mammographic or MRI finding. Some indications for a whole-breast examination include the search for satellite lesions and lymph node involvement in a patient with a known cancer or suspicious lesion to assist staging. In addition, sonography can canvas the breast to evaluate implant integrity.
As a supplement to mammography, a bilateral whole-breast US screening examination is warranted for some women with compromised mammograms due to high breast density and for women at high risk for breast cancer who are not the MRI candidates (or unable to easily access breast MRI testing). Screening US can be performed using a handheld transducer or an automated whole-breast system.
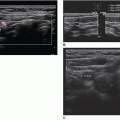
For a breast US examination, the patient is properly positioned, and a warm scanning gel is applied to the skin. The breast is methodically surveyed in overlapping orthogonal (perpendicular) scan planes with special attention paid to the areas of concern. Orthogonal scan planes include sagittal (longitudinal) and transverse, or radial and antiradial image planes (
Fig. 18-8). Radial and antiradial scan planes are of particular value during breast imaging. Radial scans better follow lobar anatomy and are often best for examining major lactiferous ducts or for documenting mass location relative to the nipple. Scanning the entire volume of a solid breast mass in both radial and antiradial planes can increase detection of tumor extension coursing toward the nipple within a major duct or branching outward into smaller ducts.
The skin overlying a palpable lump can be marked before scanning or the location confirmed during the examination.
Echopalpation (
sonopalpation) refers to concurrent palpation of a region of interest while scanning.
10,15 For example, the sonographer secures the palpable area between two fingers while scanning directly over the lump. This allows direct correlation of clinical and sonographic findings. Alternatively, the sonographer can place a thin marker producing a shadowing artifact (e.g., opened metal paper clip) between the transducer and palpable finding. Images are taken with and without this “marker” to correlate and document the location of the palpable finding. The image should be annotated as the “palpable” area of interest.
The use of an acoustic standoff between the transducer and the breast improves delineation of the skin layer and very superficial breast masses by optimizing near-field focusing and reducing artifacts. In most cases, extra scanning gel is a sufficient standoff. When a standoff pad is used, the thickness of the acoustic offset should not exceed 1 cm so that the focal plane (elevation focus) is not shifted out of the breast.
10Scanning with a variety of beam angles is needed to fully evaluate the margin characteristics of a breast mass. This can be accomplished by rocking and angling the transducer (heel-toe maneuver) and by using spatial compounding.
10 Enlarging the image to the region of interest and focusing at the level of the mass allow better inspection of mass features.
During the breast examination, applying variable amounts of transducer pressure to compress the underlying tissues is beneficial for a variety of reasons. Compression decreases breast thickness for better sound penetration by higher frequencies. Applying transducer pressure flattens Cooper ligaments to reduce critical angle shadowing. Compression will also reduce or eliminate false acoustic shadowing beneath a benign lesion or scar tissue, whereas malignant shadowing tends to persist. Alternating the application and release of transducer pressure (ballottement) helps assess the compressibility and mobility of breast masses, as well as the movement of internal echoes in complicated cysts and dilated ducts. In contrast, too much transducer pressure is detrimental during Doppler evaluation because vessel compression can reduce or ablate blood flow.
Positional changes can be advantageous when trying to shift internal debris, fluid-debris levels, or fat-fluid levels within a complicated cystic mass, or move a dependent milk-of-calcium sediment layer.
Key lesions should be documented with and without caliper measurements.
4,16 Minimally, the maximal dimensions of a mass should be recorded in at least two planes (orthogonal planes preferred). Maximal dimensions, rather than mean diameter, correlate better with mammographic measurements. Obtaining measurements in three dimensions (maximal length, height, and width) allows for more accurate comparison of lesion size, or calculation of mass volume, for follow-up imaging or after intervention. Heightto-width ratios can also be calculated when assessing benign and malignant imaging characteristics.
Obtaining a comparison scan of the opposite breast in the same region can be helpful when sorting out questionable findings and helps to contrast inflammatory or traumatic changes with the normal breast tissue. Side-to-side comparison is also helpful when assessing axillary nodes.
Based on site protocol and reason for the examination, representative images are acquired of the region of interest, or expanded to document each breast quadrant, the subareolar region, and the axilla, when indicated. Important findings are documented.
Minimum documentation of a handheld, whole-breast screening US examination that is negative for mass detection should include a representative view of each breast quadrant (at the same level) and the retroareolar region. These can be taken in a single scan plane, such as the radial plane.
10,18Images are appropriately labeled, interpreted, and archived. Most digital picture archiving and communication system (PACS) can archive dynamic cine clips, showing full sweeps of the breast tissue, pathologic conditions, and needle placement during interventional examinations.
Breast sonograms should document the facility’s name and location, the examination date, the patient’s name, date of birth and/or identification number, and the initials (or another notation) as to the sonographer/physician performing the examination.
Accurate labeling of each image should include side (right or left), transducer location and scan plane, and mass location in the breast, in order to accurately document findings for the interpreting physician. Proper image annotation is also important for follow-up comparison and to relocate a mass before intervention.
Over the years, a variety of labeling methods have been used to annotate breast sonograms, including the quadrant, clockface, and 1-2-3: A-B-C methods (
Figs. 18-9 and
18-10). Current ACR practice parameters state that the location of a breast lesion on the sonogram should be annotated using the clockface position, distance in centimeters from the nipple (not the areola), and the transducer orientation.
16 Many systems provide a breast body marker for additional documentation of transducer position and scan plane. One way to annotate a right breast mass located 5 cm from the nipple in the radial scan plane is “RT 9:00 5 CM FN RAD.” Most breast imaging transducers have a footprint of 3.8 to 5 cm, which is helpful as a measuring guide. Using a ruler or placing a piece of tape with centimeter marks along the
side the transducer helps to estimate mass-nipple distance. Some new breast transducers are now manufactured with centimeter marks embedded along the side of the transducer. On occasion, two closely positioned masses may reside in the same scan frame. In such situations, an additional measurement of the distance from the skin to the mass (mass depth) can help differentiate these lesions in the final report.
The “1-2-3: A-B-C” labeling method has been used to note the relative distance of a mass from the nipple, as well as the depth within the breast.
9,10 This method also had value when relocating a mass, especially when intervention was to be done at a different facility. Some facilities still elect to record the depth of a lesion using the A-B-C method in addition to the more current ACR annotation recommendations.
Specialty Techniques
Most current breast handheld sonography systems have split-screen capabilities, extended field-of-view (EFOV), or convex linear (trapezoidal) 2D imaging features that allow larger spans of breast anatomy and masses to be displayed and measured.
Harmonic imaging and spatial compounding are now common equipment features that improve contrast and spatial resolution during breast imaging. These techniques reduce image artifacts (e.g., speckle, noise, reverberation) and show better margin delineation, which help make subtle masses, such as small isoechoic lesions, more apparent on the sonogram. Spatial compounding creates a single real-time image from multiple scan angles and improves margin delineation. Besides reducing detrimental image artifacts, spatial compounding may also reduce demonstration of helpful attenuation artifacts. The sonographer should be aware that enhancement beneath a small cyst or subtle shadowing from a cancer may be reduced or eliminated at higher levels of spatial compounding yet be apparent on conventional imaging. Therefore, scanning a region of interest with and without spatial compounding may be necessary.
Many newer generation scanners have a system control that the sonographer can use to adjust the speed of sound, especially when imaging the fatty breast. Sound travels slower in fat than in soft tissues. Applying speed-of-sound correction can improve spatial and contrast resolution for better image quality. Improvements include better depiction of mass margins, tissue interfaces, and calcifications.
3D imaging provides surface or volume rendering of breast masses that can be further manipulated after completion of the scan. A volume data set can also be viewed in multiple static image planes (multiplanar), which is particularly helpful when assessing margin and wall characteristics of solid masses and complex cysts. 3D US shows breast masses in three orthogonal planes. Compared with conventional 2D imaging, the additional coronal plane is of particular value when analyzing the growth pattern of masses and effects on surrounding tissues. Reconstructed coronal scans better illustrate malignant features such as spiculation (
Fig. 18-11) shown as a “retraction/star” pattern, compared with a “compression” pattern seen with more circumscribed benign masses.
25 Real-time 3D imaging (4D) may be of value during guidance procedures.
SONOGRAPHIC-MAMMOGRAPHIC CORRELATION
6,7,15Breast sonography usually follows clinical and mammographic examinations with the hope of providing more specific diagnostic information for appropriate patient management. The breast sonographer must have at least a basic understanding of mammography in order to properly correlate sonographic with mammographic findings.
Table 18-3 compares the tissue densities and echogenicities of common breast structures seen on mammography and sonography.
Mammography is performed on asymptomatic patients to screen for occult breast cancer or on symptomatic patients to diagnose a clinical concern. The standard of care has been 2D full-field digital mammography (FFDM). However, many sites also offer 3D mammography, known as digital breast tomosynthesis (DBT), which allows breast tissue to be additionally reviewed in layers besides standard screening views.
A 2D mammogram is a superimposed image of all breast tissue compressed between the X-ray image receptor and a compression plate. Images are labeled for the specific view and breast side (laterality). Standard mammographic screening projections are the craniocaudal (CC) and the mediolateral oblique (MLO) views (
Fig. 18-12). Besides these standard views, additional views (e.g., spot compression, magnification, 90-degree lateral) may be obtained during a diagnostic mammogram to better assess a palpable mass or to further examine an abnormality found on the screening examination.
For the mammographic CC view, the X-ray tube is oriented vertically over the breast with the patient upright. The breast is positioned so that the X-ray beam enters the superior (cranial) breast and exits the inferior (caudal) breast. The side marker is always placed by the lateral breast. With the nipple centered, this view demonstrates the medial, central, and lateral breast.
The MLO view shows breast tissue from the axilla to the inframammary fold and includes a portion of the pectoralis muscle and lower axilla. The nipple is seen in profile. The
side marker is placed near the axilla at the outer breast. Depending on body habitus, the plane of the image receptor for the MLO view is approximately 45 degrees (±15 degrees) from horizontal to be parallel with the plane of the pectoralis muscle. Unlike a true 90-degree lateral projection, the X-ray beam passes from the superomedial to the inferolateral breast for the MLO projection. For screening purposes, the MLO view shows the greatest amount of breast tissue in a single view. This projection best images the UOQ and axillary tail but may exclude some posteromedial tissue. Lower axillary lymph nodes are often visible on this projection.
Although not a screening view, a true 90-degree lateral projection shows a true profile of the breast and accurately shows the superior-to-inferior location of masses relative to the posterior nipple line. However, this view restricts seeing the axillary region. This mammogram view is helpful for mass localization before biopsy and for demonstration of air-fluid or fat-fluid levels or dependent milk-of-calcium sediment.
The size, shape, location, and surrounding tissue density need to be compared when correlating a breast mass on mammography and sonography.
9,10 Differences in technique, patient positioning, and direction of tissue compression between the mammogram and the sonogram can affect the relative location, orientation, and shape of a breast mass when correlating imaging findings. The margin characteristics and tissues surrounding a mass should be compared between modality images for concordance (e.g., mass surrounded by fat). During mammographic positioning, breast tissues (and masses) are pulled away from the chest wall. During sonography, structures are pushed toward the chest wall by transducer compression in an anteroposterior (AP) direction, making breast layers appear thinner. Taking this into account, a mass that abuts the anterior mammary layer on the mammogram should still correlate with that location on the sonogram. Compression can also alter the shape and orientation of some masses when correlating findings between modalities.
The radiologist can assist the sonographer when triangulating the true location of a breast mass seen on a mammogram before US scanning because patient positioning is different. The CC view shows the lateral, central, or medial location of a mass with minimal rotation and best correlates with the US location. Because of the oblique path of the X-ray beam for the MLO view, a mass may project higher or lower on the mammogram than its actual location in the breast. A medial mass can actually lie higher, whereas a lateral mass can lie lower in the breast than where it is shown on the MLO view. This is especially true for more peripheral
lesions near 3:00 or 9:00. This must be taken into account when performing the US scan and may necessitate scanning a larger wedge of tissue to locate the mass. Remembering the acronym “MULD” for
medial-up and
lateral down will help the sonographer remember this concept during sonomammographic correlation. Generally, a more centrally located breast mass (near 12:00, subareolar, or 6:00) is more accurately shown as to its true location on standard screening mammogram views.
Real-time scanning an entire breast hemisphere is often needed to locate a mass seen on only one mammographic view. For example, a mass shown only on the CC view in the lateral breast may necessitate scanning the full outer hemisphere of the breast.
With the patient supine, it is occasionally difficult to be certain whether a mass seen by sonography is the same mass demonstrated by mammography, especially in larger breasted women. By scanning the patient in a position that simulates the radiographic projection, the sonographic location of a mass can be more accurately correlated, although breast tissues will be less compressed. To simulate the CC projection during the sonography examination, the patient can sit upright and use her opposite hand to lift the lower surface of the breast so the nipple is in profile.
14,15 This technique can be helpful to locate a mass seen only on the CC view.
SONOGRAPHIC DESCRIPTORS AND CLASSIFICATION OF FINDINGS
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15 and
16,26The ACR originally developed the Breast Imaging Reporting and Data System (BI-RADS) to standardize the reporting of mammographic findings, as well as classifying findings into risk categories for cancer.
4 The BI-RAD system is now also applied to the classification of sonography and MRI findings. Recommendations for patient management follow the level of risk assessment (
Table 18-4).
Breast patients are often referred for US examination based on a screening mammogram report that states the following: BI-RADS Category 0: incomplete; needs additional imaging evaluation. In such cases, the radiologist will require “more information” before assigning a final risk category. Often, these are cases when a mass or focal asymmetry is present on the mammogram and sonography is needed to further characterize the mass as solid or cystic and to provide other diagnostic imaging features that may favor a benign versus a suspicious process.
The ACR published a “BI-RADS Breast Ultrasound Lexicon” to promote the use of more consistent terminology when describing and reporting sonographic findings (
Table 18-5).
4,16 When applicable, similar terms are applied to mammographic and sonographic features. The ACR BI-RADS lexicon also includes descriptions of tissue composition to be reported with screening breast US examinations. Breast tissue composition on US can appear homogeneously fatty, or display homogeneous echogenic fibroglandular tissue with little surrounding fat, or show a more heterogeneous echo pattern.
When a mass is demonstrated by sonography, its imaging characteristics must be carefully examined, as well as any effects on surrounding tissues. A mass occupies space and must be confirmed in orthogonal US scan planes. Real-time imaging facilitates complete inspection of a mass. For lesion analysis, primary US features include mass shape, orientation in the breast relative to the skin line, margin characteristics, internal echo pattern, and posterior acoustic features. Mass features of most importance for cancer risk assessment are shape, orientation, and margin characteristics.
4,9,10,16Sonographic features most characteristics of a benign solid breast mass are oval shape, a parallel orientation relative to the skin (wider-than-tall, horizontally positioned), and a smoothly circumscribed margin. A circumscribed mass shows sharp demarcation from surrounding tissues along its entire margin. An oval mass is elliptical or egg shaped and may display two or three surface undulations, alternatively referred to as gentle lobulation or macrolobulation. Using the BI-RADS US lexicon, “irregular” is a descriptor of shape rather than margin. Unlike cysts, solid breast masses are less often round. An oval, parallel-oriented lesion will have a greater length than height, a feature referred to as “wider-than-tall.”
9,10 Benign solid masses tend to be mildly
hypoechoic or isoechoic compared with fat and show normal sound transmission (no posterior features) or mild enhancement. A purely hyperechoic lesion or focal region of hyperechoic fibrous stromal tissue in the area of clinical concern also favors a benign diagnosis.
Sonographic features most suspicious for malignancy include an irregular shape, nonparallel orientation (taller-than-wide, vertically positioned), and a margin that is not circumscribed along all, or any portion, of the mass. Round masses are also considered “not parallel” in orientation. Margins that are “not circumscribed” may be indistinct, angular, microlobulated, or spiculated. An indistinct margin is ill-defined and shows no clear demarcation between the lesion’s border and surrounding tissues. The presence of a thick echogenic rim can also make margins indistinct. In addition, a cancer may show a combination of circumscribed and noncircumscribed features.
Echogenicity alone is a less specific criterion when predicting cancer because most benign and malignant solid masses are hypoechoic compared with normal subcutaneous fat. However, a markedly hypoechoic mass is a suspicious finding, especially when combined with other worrisome imaging features.
Masses attenuate sound differently. Shadowing is a suspicious posterior feature of a solid mass and can, in part, reflect fibrotic changes within and around the lesion (e.g., desmoplasia), but may also occur with certain benign processes. In addition, higher grade, more cellular breast cancers can show sound enhancement.
A heterogeneous echo pattern shows as mixture of internal echogenicities and has less validity in predicting cancer.
4 A complex cystic and solid mass displays both anechoic (cystic or fluid) and echogenic (solid) components and can be worrisome in the absence of known infection or trauma.
When detected by US, breast calcifications are reported as calcifications in a mass, calcifications outside a mass, or intraductal calcifications. Calcifications can be benign or malignant and are better detected and characterized as to size and distribution by mammography. Because of their tiny size (<0.5 mm), microcalcifications do not shadow on US because of smaller than the sound beam width and are typically best seen within a hypoechoic breast mass. A breast mass with microcalcifications is suspicious for cancer. However, newer high-resolution transducers and equipment processing features have made microcalcifications more discernible with US imaging. Macrocalcifications are typically benign.
Associated features are listed in the ACR lexicon. These include changes in adjacent tissues or structures owing to the presence of a breast mass or abnormality. Associated changes in the skin, tissues planes, Cooper ligaments, ductal structures, and vascularity provide additional clues when differentiating benign from malignant growth patterns. Sometimes, a focal area of architectural distortion or tissue disruption is evident without a focal mass. A benign mass typically compresses, rather than disrupts, adjacent tissues. Suspicious findings include straightening or thickening of Cooper ligaments, obliteration of tissue planes, or aberrations in ductal patterns. The presence of intraductal extension from a tumor should be sought because it impacts surgical management.
7,10Duct changes that occur in the breast include cystic dilatation, irregular caliber and/or arborization, ductal distortion by a mass, ductal extension to or from a suspicious mass, or an intraductal mass, thrombus, or debris. Skin changes can manifest as areas of thickening or retraction can indicate underlying malignancy, inflammatory cancer, or be related to benign inflammation, prior trauma, or radiation. Breast edema produces increased echogenicity of affected tissues and may have a reticulated appearance from dilated lymphatics and interstitial fluid. Tissue edema can have benign causes (e.g., mastitis, congestive heart failure, irradiation) but could also manifest with malignancy (e.g., inflammatory carcinoma, lymphoma).
4,14Elasticity assessment of breast masses is a recent addition to the lexicon, although not available at all US facilities. Elastography evaluates the stiffness of breast lesions relative to adjacent tissues. Similar to clinical palpation, benign masses tend to be soft or compressible, whereas malignant masses tend to be hard or stiff when assessed by elastography methods. Using the ACR lexicon, stiffness characteristics are reported as
soft,
intermediate, or
hard.4 Elastography is performed in conjunction with 2D US imaging. Greater reliance is given to the morphologic features seen on 2D imaging for equivocal findings.
Breast cancers typically show several suspicious imaging findings, but not always. Some cancers resemble a benign mass, whereas some benign masses and inflammatory and traumatic changes mimic cancer, so close correlation with clinical history and related imaging examinations is important. Because mass characterization is also affected by technical factors, adherence to proper scanning technique and equipment setup is critical, as well as recognition of diagnostic pitfalls.
Special cases are listed in the ACR BI-RADS US lexicon that include conditions with a unique diagnosis or display specific findings, including
simple cyst, clustered microcysts, complicated cysts, mass in or on the skin, foreign body including implants, lymph nodes, vascular abnormalities, postsurgical fluid collections, and
fat necrosis.4 These are discussed later in this chapter, along with further discussion on solid breast mass assessment and elastography.
BREAST DISEASE AND TISSUE VARIATIONS
A variety of physiologic and pathologic changes affect the breast, many of which have features detectable by imaging techniques. Many of these conditions are reviewed in this chapter.
Sonographic Assessment of Cystic Breast Masses
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14 and
15,27During the sonographic examination of a breast mass, the first step is to determine whether the mass is cystic or solid. Cystic breast masses can be described as simple cysts, clustered microcysts, complicated cysts, or complex cystic and solid masses based on imaging findings. Classifying a simple breast cyst is easy when diagnostic criteria are met but can be confusing when cyst fluid is complicated by cellular debris, hemorrhage, or infection. Cystic masses that contain solid elements add to diagnostic uncertainty and require additional workup to determine their benign or, possibly, malignant nature. Clinical history and correlative imaging factor into making a differential diagnosis.
Simple Cyst
True cysts are epithelial-lined, fluid-filled masses. Most breast cysts are related to fibrocystic change (FCC) and develop over time from progressive dilatation of small ducts within an obstructed TDLU. Cysts are the most common cause of breast lumps in women aged 35 to 50 years. They can be single or multiple in number and range from millimeters to several centimeters in size. Ductal secretions and fluid reabsorption contribute to variability in cyst size. With time, most cysts regress or involute. Cysts are uncommon in young or elderly women. However, in postmenopausal women receiving HRT, a cyst may develop, persist, or enlarge.
Signs and Symptoms
Small and flaccid cysts are often asymptomatic. Tense or enlarging cysts are usually palpable and may be tender. Palpable cysts are typically round or oval, smooth, and freely mobile. Most cysts are compressible unless tense.
Mammographic Features
On a mammogram, cysts appear as round or oval, smoothly marginated water density masses that are often equal in density to the surrounding parenchyma. These circumscribed masses compress adjacent tissues and are encircled by a thin radiolucent rim, referred to as the
halo sign (
Fig. 18-13A). Although findings appear benign, mammography alone cannot tell whether a circumscribed water density lesion is cystic or solid, so a sonography examination is recommended for mass characterization.
Sonographic Features of a Simple Cyst
The accurate diagnosis of a simple breast cyst is a key role of sonography and is important for subsequent patient management. Diagnostic criteria for a simple cyst are summarized in
Table 18-6. Diagnostic US findings include a round or oval shape, a thin-walled circumscribed wall, absent internal echoes (anechoic), and posterior enhancement. Thin refractive edge shadowing is often apparent unless scanning with spatial compounding. In addition, transducer pressure can show the compressibility and mobility of a cyst. No color Doppler signal will be generated by the fluid-filled mass. When strict criteria are met, the diagnosis of a simple cyst can be made with a high level of confidence, allowing for a BI-RADS 2 (benign) ACR classification (
Fig. 18-13B). In such cases, no intervention or follow-up is required unless the cyst is symptomatic or enlarges, or if the presence of multiple cysts reduces mammographic efficacy for cancer screening.
Technical Considerations
Appropriate equipment settings and scanning technique are critical to ensure diagnostic accuracy. Angulation of the sound beam allows all walls to be visualized, helping to exclude wall thickening or an intracystic mass. Proper focusing and the use of spatial compounding and/or harmonic imaging enhance margin delineation and help reduce internal artifacts. A true simple cyst should be echo free at normal gain settings and retain a crisp back wall at low gain. Sound enhancement may diminish when a cyst is small, viscous, lies deep near the muscle, or can be related to technical factors (e.g., poor focusing, use of spatial compounding).
Clustered microcysts describes a grouping of tiny cysts (each <2 to 3 mm) separated by thin (<0.5 mm) intervening septations or walls. This finding represents cystic dilatation of the tiny ductules (acini) within an enlarged breast lobule. At times, the surface may appear microlobulated, somewhat like a cluster of grapes, yet the margin remains distinct. Occasionally, tiny, dependent, hyperechoic foci may be evident, reflecting
milk of calcium. Microcyst clusters with thin septations, and no solid components, are typically benign
4,5 (
Fig. 18-14). The presence of thick isoechoic septations or solid components increases the risk of an intracystic papilloma or malignancy.
Complicated Cysts versus Complex Cystic and Solid Masses
Some cystic breast masses contain internal echoes or wall changes that are evident with high-resolution sonographic systems. These masses do not meet “simple cyst” criteria and are, instead, classified as complicated cysts or complex cystic and solid masses. A “nonsimple” breast cyst should be evaluated for signs of acute inflammation or infection and for more worrisome signs of neoplastic change. Possible causes of real echoes within a cystic breast mass are listed in
Table 18-7.
9,10Circumscribed, thin-walled, complicated cysts are typically benign and often related to FCCs. These are frequently nonpalpable incidental findings and often coexist with simple cysts. Only rarely is a true intracystic tumor present, which may be benign or malignant.
Sonographic Features
Sonography of a nonsimple cystic mass involves assessment of the cyst wall, internal echo pattern, movement of internal echoes, and surrounding tissues (
Table 18-8). Color- or power-mode Doppler assesses for vascularity of the cyst wall and internal contents.
Complicated cysts represent breast cysts with internal debris without a discrete solid component.
4,9,10,27 Otherwise, these cysts maintain a thin, circumscribed wall and typically show distal acoustic enhancement. Echoes within complicated breast cysts can present as a fluid-debris level, a fat-fluid level, or homogeneous low-level internal echoes (
Fig. 18-15 A-C). The mobility of the internal echoes and fat-fluid levels can be assessed by changing the patient’s position or applying and releasing transducer pressure over the mass. Increasing the system’s transmit power or applying Doppler can induce lightweight echoes within a cyst (e.g., cholesterol crystals) to move or “stream” away from the transducer.
9,10 The internal echoes may appear to scintillate. A color flash generated by these moving particles is termed “color streaking” when applying color- or power-mode Doppler to the cyst contents. The nondependent echogenic layer of a fat-fluid level can take 5 minutes or longer to shift with positional changes.
9,10
Circumscribed, thin-walled, complicated cysts, especially when coexist with other simple cysts, or bilateral cysts, favor a benign diagnosis, such as that with FCC.
An “acorn cyst” describes a cyst that displays a nondependent echogenic layer. A cyst with a fat-fluid level can have this appearance when the echogenic lipid layer floats anteriorly. Showing movement of this fat layer helps to differentiate this finding from a crescent-like layer of echogenic papillary apocrine metaplasia (PAM) that is fixed to the wall. When using power Doppler fremitus, a true papillary lesion within a cyst will vibrate and transmit the fremitus artifact, whereas a nonattached fat layer will not.
9,10Homogeneous internal echoes within a complicated cyst can mimic a solid mass. Inspissated foam or gel cysts are often filled with diffuse low-level internal echoes. Sources of these internal echoes include proteinaceous or fatty material, or PAM.
9,10 Compressibility and lack of internal Doppler vascularity can help differentiate a “solid-appearing” complicated cyst from a true solid mass. Sonoelastography can sometimes
be useful in this clinical setting. Aspiration may be warranted to differentiate some complicated cysts from a solid mass.
Cysts complicated by inflammation or infection affect the cyst wall and internal contents. Sonographic features worrisome for acute inflammation or infection include uniform isoechoic wall thickening, hyperemia of the cyst wall, and, possibly, internal fluid-debris level (
Fig. 18-16).
9,10 Heavier white blood cells (WBCs) or tumefactive sludge tend to settle dependently within an infected cyst and can take longer to shift with positional changes. Color- or power-mode Doppler can display blood vessels coursing along the wall of a hyperemic inflamed cyst, as opposed to tumoral vessels coursing across the cyst wall. Supportive clinical features of inflammation include focal pain, tenderness, and, possibly, redness and thickening of the overlying skin. Fibrotic cyst wall thickening from prior inflammation typically lacks hyperemic change.
Older hemorrhagic, infected, or oil cysts may develop thin-walled (eggshell) calcification, causing acoustic shadowing that may obscure all or a portion of the cyst contents.
Complex
cystic and solid mass, rather than complex cyst, is the preferred ACR BI-RADS term used to describe a mass that displays both anechoic (cystic or fluid) and discrete echogenic (solid) components.
4 Although most lesions are benign, such findings increase suspicion for neoplastic change. Worrisome sonographic findings include wall thickening (≥0.5 mm), thick internal septations (≥0.5 mm), intracystic mural nodule, or a mixed cystic/solid mass. Both the outer wall and any solid intracystic component should be evaluated for suspicious imaging features (e.g., noncircumscribed margins, duct extension, or invasion past the cyst wall).
10 Echoes from benign proliferative changes along the cyst wall or from an intramural tumor will not shift with positional changes. Doppler confirmation of blood flow within an eccentrically thickened wall or a fibrovascular stalk suggests neoplastic growth rather than PAM. A true intramural neoplasm, such as an intracystic papilloma or carcinoma, is rare. Ductal carcinoma in situ (DCIS) and necrotic neoplasms can also present as complex cystic and solid lesion by sonography.
Certain benign masses and fluid collections can image as complex cystic and solid masses. Besides papillary lesions, these include galactocele, fat necrosis, phyllodes tumor with prominent cystic elements, hematoma, and abscess. Because suspicious findings necessitate intervention, correlation with clinical history and mammographic findings is important if biopsy is deferred.
Technical Considerations
Before characterizing a cyst as complicated or complex, technical factors and sound beam focusing must be optimized to eliminate as many “false echoes” from artifacts as possible. Common detrimental artifacts include reverberation, slice thickness artifacts, and clutter. The gain settings should be set high enough to visualize true echoes within cyst fluid without introducing false echoes. Harmonics and spatial compounding can help reduce troublesome artifacts.
Assessment and Treatment
Patient history and other imaging findings are helpful when considering a differential diagnosis for a complicated or complex cystic mass. In most cases, well-circumscribed, thin-walled complicated cysts are benign and part of the spectrum of FCCs.
Classification of complicated/complex cysts into BI-RADS categories is reported in the literature.
4,5,10 A benign (BI-RADS 2) classification can be appropriate for asymptomatic, thin-walled, circumscribed, complicated cysts with mobile internal echoes or fluid-debris levels, especially in the setting of bilateral multiple breast cysts. When discovered on a baseline sonography examination or as an incidental finding, asymptomatic complicated cysts with homogeneous internal echoes and clustered microcysts are generally classified as “probably benign.” In such cases, the patient may be offered short-interval follow-up.
Aspiration of a benign simple cyst is not needed unless the patient is symptomatic (e.g., discomfort, tense palpable cyst), or when numerous cysts compromise mammographic quality. Benign-appearing aspirates that are green, yellow, clear, or black can be discarded.
9 Aspiration or core biopsy may be needed to differentiate an echo-filled complicated cyst from a solid mass. Cysts filled with PAM may not aspirate.
9 Aspiration is warranted for symptomatic complicated cysts. A bloody aspirate should undergo cytologic analysis to exclude malignant cells. Cysts that recur after aspiration or cannot be completely aspirated may require excision. Aspiration can be diagnostic and therapeutic for cysts suspected to be acutely inflamed or infected. Purulent fluid aspirated from a symptomatic cyst should be sent for Gram stain and culture to determine whether the cyst is infected or just inflamed.
9,10With suspicious complex cystic and solid lesions (BI-RADS 4), large core or sonography-directed vacuum-assisted biopsy (VAB) is preferred to cyst aspiration because the cyst wall and solid components are of histologic importance. Placement of a marker clip serves as a landmark should excision be required.
Benign Cystic Masses and Related Conditions
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14 and
15
Fibrocystic Change
FCC is the most common benign diffuse breast condition. Women are typically most symptomatic during late reproductive life (35 to 55 years). FCC is not considered a
“disease” but rather signifies several changes considered to be aberrations of normal development and involution. With aging, female estrogen levels can predominate as menstrual cycles become irregular and ovulatory patterns change. This hormonal imbalance helps induce changes that primarily affect the ducts, lobules, and connective tissues of the TDLUs. Key features of FCC include epithelial hyperplasia, adenosis, stromal fibrosis, and cyst formation. Most FCC findings are nonproliferative and do not increase breast cancer risk.
Signs and Symptoms
At least half of adult women develop some symptoms related to FCC, including breast tenderness or pain, fullness, and nodularity. Symptoms are usually bilateral and often increase before menses. Nipple discharge, if present, can be from more than one duct orifice and involve both breasts and is often yellow or green in color. Symptoms diminish after menopause unless, but may persist if, a woman is on HRT.
Mammographic Features
Common features of FCC include the presence of bilateral, rounded, circumscribed water density masses, and benign scattered round/punctate parenchymal calcifications. In some patients, milk-of-calcium sediment within microcysts appears as “teacup-shaped” calcifications. Multiple enlarged lobules (adenosis) can give the parenchyma a “snowflake” appearance. Increased radiodensity of the breast parenchyma can hinder detection of some cysts and lobular changes. Certain benign FCC alterations such as sclerosing adenosis, radial scars, and focal fibrosis can occasionally produce suspicious imaging findings.
Sonographic Features
The most common sonographic finding is the presence of multiple cysts of varying sizes in both breasts (
Fig. 18-17). Some complicated and septated cysts fall within the spectrum of FCC. When seen, clustered microcysts may show tiny hyperechoic foci, reflecting milk of calcium. Stromal fibrosis can increase parenchymal echogenicity and sound attenuation. The fibroglandular tissue may have a basket-weave appearance with ductal prominence. High-resolution sonography may detect small solid nodules related to FCC. Enlarged lobules (adenosis) can measure up to 7 mm and appear as isoechoic or hypoechoic solid nodules. Nodular adenosis may appear as a circumscribed mass. Sclerosing adenosis may display an irregular shape, microlobulation, or microcalcifications, making differentiation from cancer difficult.
Galactocele
A galactocele is a milky cyst caused by obstruction of a lactiferous duct in the pregnant or lactating woman. This retention cyst tends to develop after abrupt cessation or suppression of lactation and contains fat, proteins, and lactose. A subareolar location is common, but galactoceles also occur in a peripheral TDLU. A galactocele may persist past the lactation period, undergo oily transformation, and become a lipid (oil) cyst. Rupture of an infected galactocele can lead to mastitis and subsequent abscess formation.
Signs and Symptoms
A galactocele is the most common benign mass to develop in a lactating patient. The presenting mass is usually firm but mobile. Tenderness or mastitis can accompany an infected galactocele.
Mammographic Features
A galactocele appears as a circumscribed mass containing radiolucent (fat density) material, which is a benign feature. Radiolucency varies with the fat and protein content. Increased parenchymal density of the lactating breast can hinder mammographic detection of some masses. A thin rim of wall calcification may occur with older lesions.
Sonographic Features
This round or oval, circumscribed mass can be unilocular or multilocular in appearance. Although galactoceles can be anechoic, variable amounts of internal echoes reflect from milk-laden contents (
Fig. 18-18), often presenting as a complicated cyst. A fluid-fat level can be present and slowly shifts with positional changes. Doppler confirms the absence of internal blood flow. Posterior enhancement may be less than that seen with a simple cyst. Rim calcification, if present, can impede sound transmission. Associated
findings in the lactating patient are prominent glandular tissue and dilated ducts.
Differential Diagnosis
Oil cysts and other complicated cysts can have similar sonographic appearances. If mastitis is present, an infected cyst or localized abscess is considered. A tender mass with a thick hyperemic wall can indicate an infected galactocele.
Sebaceous Cyst and Epidermal Inclusion Cyst
Sebaceous and epidermal cysts are small, benign, skin appendage masses that result from obstructed sebaceous glands or hair follicles and contain sebum or keratin. The superficial location is a clue to their origin, residing within or just beneath the dermal layer of the skin. Sebaceous cysts are often located at the inferior or medial margins of the breast or near the axilla. Occasionally, epidermal cysts form after breast trauma (e.g., needle biopsy, reduction mammoplasty). Retention cysts can also develop within the Montgomery glands of the areola. Infected or ruptured sebaceous cysts can lead to abscess formation.
Signs and Symptoms
Nodules are palpable because of their superficial location. The skin pore overlying the obstructed gland may be darkened. Skin reddening and tenderness overlying the cyst can indicate infection.
Sonographic Features
Sebaceous and epidermal cysts are round or oval in shape with smooth, thin-walled, circumscribed margins. The cyst can be completely or partially located within the dermis or project beneath the skin into the subcutaneous fat (
Fig. 18-19). Focal skin thickening usually accompanies the cyst. When a cyst projects into the subcutaneous fat, the surrounding dermis can have a “claw-like” appearance. Although these cysts can be anechoic, the greasy or waxy contents often generate low- to medium-level internal echoes, fluid-fat levels, or produce a multilaminated appearance. Posterior acoustic enhancement is typically preserved. Slight angulation of the sound beam can improve detection of a narrow, hypoechoic, excretory duct or inflamed hair follicle that extends from the cyst to the skin surface (
Fig. 18-20). Color Doppler reveals no internal blood flow. Cysts of skin origin can be classified as BI-RADS 2 benign lesions.
4Signs of cyst infection or abscess formation are sought when tenderness and skin reddening are present. Over time, sebaceous cysts may develop wall calcification with associated acoustic shadowing.
Technical Considerations
These superficial lesions are best delineated utilizing a very high-frequency transducer, an acoustic offset (such as extra scan gel), and proper near-field focusing. A small footprint (hockey stick) linear transducer can optimize imaging small superficial cysts.
Benign Inflammatory Conditions
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14 and
15
Mastitis
Mastitis, or inflammation of the breast, presents most often during pregnancy and lactation but can affect women at any stage of life. Causes are varied. Nonlactational forms of mastitis may result from an infected or ruptured cyst, a ruptured ectatic duct, postsurgical infection, periductal inflammation, or due to granulomatous conditions.
Puerperal mastitis represents breast inflammation related to lactation. It is the most frequent cause of acute mastitis. Bacteria, typically Staphylococcus aureus, can enter the ducts through a skin abrasion or cracked nipple or ascend via the ducts. Obstructed lactiferous ducts are susceptible to infection. Dilated infected ducts can show thick isoechoic walls and internal echoes from inspissated milk. With puerperal mastitis, infection can begin centrally within a lactiferous duct or develop more peripherally in a galactocele. Without appropriate treatment, an abscess may develop. Infection may potentially spread by way of the blood and lymph vessels to other parts of the breast.
Mammography is compromised in the woman with acute mastitis due to increased breast density from edema and
also from glandular proliferation related to lactation. Breast tenderness can further limit adequate tissue compression. Depending on the patient’s age and clinical history, sonography is often the initial imaging examination for patients presenting with mastitis to search for complications such as abscess formation.
Mammary duct ectasia and periductal (plasma cell) mastitis primarily affects subareolar structures. This uncommon, chronic, nonlactational form of mastitis presents closer to menopause, although younger women with congenital nipple inversion are also at risk. Symptoms can include subareolar nodularity, a thick sticky nipple discharge, nipple retraction, and discomfort. Clinical features can mimic cancer; however, the ductal ectasia-periductal mastitis complex is usually bilateral in presentation. Subareolar ducts become distended with thick secretions and debris. Irritation and damage to the duct wall can allow secretions to leak into surrounding tissues, inciting periductal inflammation. Periductal fibrosis may follow and eventually shorten the ducts, resulting in nipple retraction. Mammogram features include an increase in subareolar density from dilated ducts or from a possible abscess. Calcification can develop both within and around the affected ducts.
In patients with periductal mastitis, sonography is primarily used to determine whether the cause of subareolar nodularity is from duct ectasia, abscess, or due to a mass. Dilated mammary ducts converging toward the nipple may display thick walls and contain echogenic, inspissated secretions. Color Doppler may show increased blood flow along the duct wall. Rupture of a duct can incite formation of a subareolar or periareolar abscess that may develop fistula tracts. Over time, there can be fibrous obliteration of the duct lumen.
Abscess
Abscesses are localized areas of pus and necrotic tissue that develop in a small percentage of patients with breast infection. Most often, abscesses form beneath the nipple (subareolar), or they may develop under the skin (subcutaneous), within the gland (intramammary), or deep to the gland in front of the pectoral muscles (retromammary).
Signs and Symptoms
Acute mastitis presents with varying degrees of fever, pain, skin reddening and thickening, and a purulent discharge. An elevated WBC count supports infection. In this setting, a palpable mass can indicate an abscess. Axillary nodes may be enlarged and tender.
Sonographic Features
The sonographic appearance varies with the stage and distribution of the inflammatory process. The inflamed, thickened skin prompts a search for an underlying infected duct or fluid collection. Swollen edematous breast tissues scatter and attenuate the sound beam, requiring lower frequencies to adequately penetrate the tissues. Edema can increase the echogenicity of the subcutaneous fat, which reduces delineation of the Cooper ligaments and other tissue planes. Dilated subdermal lymphatic channels or interstitial fluid may be seen. Comparison of the inflamed breast to a similar region in the normal contralateral breast allows the sonologist to appreciate the degree of inflammatory change.
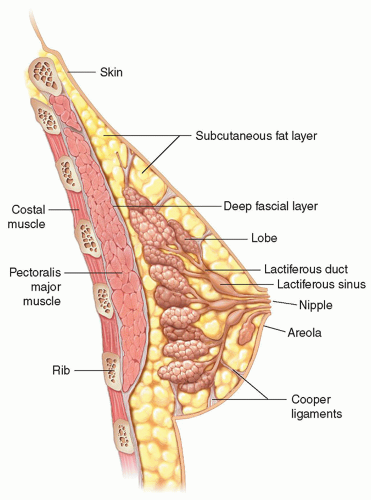








 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access